Abstract
Summary
The aim of this review is to compare the efficacy and safety of denosumab over other treatments for osteoporosis. The results of this study suggest that the safety of denosumab and its efficacy in reducing fractures is not significantly different from bisphosphonates. Denosumab was, however, more effective in increasing bone mineral density.
Introduction
This study was conducted to compare the efficacy and safety of denosumab over other pharmacological treatments for osteoporosis in individuals at risk of fracture.
Methods
Randomised controlled trials comparing denosumab with another pharmacological treatment for osteoporosis were searched in MEDLINE, EMBASE and CENTRAL. Identified articles were screened by two independent reviewers and assessed for inclusion. Data from included studies were extracted and meta-analyses were conducted using random effects models.
Results
Nine studies including a total of 4890 postmenopausal women were identified. The follow-up period varied from 12 to 24 months. In all studies except one, the comparator treatment was a bisphosphonate. There was no statistically significant difference between patients receiving denosumab and those receiving a bisphosphonate in terms of fracture risk (RR[95 % CI] = 1.15 [0.84–1.58]), adverse events (RR[95 % CI] = 0.99 [0.96–1.02]) or deaths (OR[95 % CI] = 0.58 [0.12–2.71]). Withdrawals due to adverse events were less frequent in denosumab than in other treatment groups but the difference did not reach statistical significance (OR[95 % CI] = 0.68 [0.45–1.04]). The percent change in bone mineral density at the total hip, lumbar spine, femoral neck and one-third radius was significantly higher in participants who received denosumab (e.g. mean difference [95 % CI] at the total hip: 1.06 [0.86–1.25]).
Conclusions
These results suggest that, after 12 to 24 months, the safety and efficacy of denosumab for reducing fracture risk is not significantly different from bisphosphonates despite higher gains in bone mineral density. In a clinical setting, denosumab may demonstrate greater effectiveness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a systemic skeletal disease characterised by low bone mineral density (BMD) and deterioration of bone architecture resulting in reduced bone strength and, consequently, an increased susceptibility to fracture [1]. In patients without fracture, measurement of BMD by dual energy x-ray absorptiometry (DXA) is commonly used for the diagnosis of osteoporosis. According to the World Health Organisation and the International Society for Clinical Densitometry Position, postmenopausal women and men older than 50 years with a BMD T-score of −2.5 or below have osteoporosis and those with a BMD T-score between −1 and −2.5 have low bone mass or osteopenia [2–4].
Several treatments are available for osteoporosis and fracture prevention, including bisphosphonates, teriparatide, raloxifene, and denosumab, which was approved by the Food and Drug Administration in 2010 [5]. Like bisphosphonates, denosumab inhibits bone resorption. It acts by binding to and inhibiting the effect of the receptor activator of nuclear factor KB ligand (RANKL), a protein that stimulates osteoclast activity. Denosumab is indicated in the treatment of women and men with osteoporosis at high risk for fracture. It is administered subcutaneously every 6 months at a dosage of 60 mg [6]. In the FREEDOM study, denosumab given twice yearly for 36 months has been shown to reduce the risk of new radiographic vertebral fractures by 68 %, hip fractures by 40 %, and non-vertebral fractures by 20 % when compared to placebo [7].
While the mechanism of action of denosumab differs to that of other treatments, its comparability in terms of efficacy and safety is unknown. We therefore conducted a systematic review and meta-analysis of randomised controlled trials to evaluate the efficacy and safety of denosumab compared to other approved treatments for osteoporosis, in individuals at risk of fractures or suffering from osteoporosis.
Methods
This systematic review was developed according to recommendations from The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements [8] and the Cochrane Handbook for Systematic Reviews of Interventions [9]. Eligibility criteria, outcomes, data to be extracted, risk of bias assessment and statistical analyses were determined a priori and formulated in a protocol (unpublished).
Eligibility criteria
Randomized controlled trials comparing the effect of denosumab (at any dose level) with another pharmacological treatment for osteoporosis were retained for this review if the study population included at least 80 % of men or women aged 40 years and older and if at least 80 % of participants were at risk of fracture or suffered from osteoporosis. Being at risk of fracture or suffering from osteoporosis was defined as (i) having suffered a non-traumatic fracture, (ii) having a BMD T-score lower than −1.8 (arbitrary value corresponding approximately to the midpoint of the osteopenic BMD interval (−2.5 to −1)), (iii) being diagnosed with primary osteoporosis or (iv) being considered at risk of fracture by study investigators. Studies published in any language, peer-reviewed or not, were included. Trials comparing the effect of denosumab with calcium or vitamin D or including only individuals with a specific condition other than osteoporosis were excluded.
Outcomes
Our primary outcomes were fractures (vertebral, hip or at all skeletal sites) and adverse events (all adverse events, withdrawals due to adverse events, death). Our secondary outcomes were the changes in areal BMD from baseline at the total hip, lumbar spine, femoral neck and one-third distal radius, as measured by DXA.
Search strategy
The electronic databases MEDLINE, EMBASE and Cochrane Central Register of Controlled Trials (CENTRAL) were searched from their inception through May 2015 for published studies on the effect of denosumab. The search strategy was first designed for MEDLINE (Appendix A) and then adapted for the other databases using a combination of exploded MeSH or Emtree terms and keywords related to the terms “denosumab”, “osteoporosis”, “fracture” and “low bone mass”. Highly sensitive filters were used to identify randomised controlled trials in humans [9, 10]. To identify additional studies, references from identified articles and existing reviews were screened. All references were imported into EndNote (version X7.0.2, Thomson Reuters) software and duplicates were removed.
Study selection
Based on the inclusion and exclusion criteria, two independent reviewers (CB and SJ) screened titles and abstracts of each study to determine eligibility. Both reviewers independently assessed the full text of studies classified as included or unclear for final inclusion. Disagreements were discussed and, when required, resolved by a third reviewer (JPB).
Data abstraction
Using standardized data extraction forms, piloted on two representative studies, two reviewers (CB and SJ) independently extracted data on study characteristics (e.g. design, country, inclusion and exclusion criteria, funding sources), participants’ clinical characteristics (e.g. age, gender, ethnicity, BMD T-score, prior treatment for osteoporosis), interventions and cointerventions (name, dosage, frequency, duration) and outcomes. Data not reported in an article were extracted from protocols when available. Numerical data only available from plots or images were extracted using WebPlotDigitizer (version 2.6). Authors were not contacted for missing data.
Risk of bias assessment
Two reviewers (CB and SJ) independently assessed the risk of bias of selected studies using the Cochrane Collaboration’s tool for assessing risk of bias [9]. A low, high or unclear risk of bias was assigned to each of the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and selective reporting. Protocols of studies were searched and used to better evaluate the risk of bias. An overall high risk of bias was attributed to studies in which (i) the analyses included fewer than 80 % of randomized subjects, (ii) participants were not blinded to treatment or (iii) two domains or more had a high risk of bias. Studies were considered to have an unclear overall risk of bias if (i) fewer than two domains had a high risk of bias and (ii) two domains or more had an unclear risk of bias. Otherwise, studies were considered to have a low overall risk of bias.
Statistical analyses
Meta-analyses were conducted with Review Manager (version 5.2, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012) using data reported on the longest follow-up period of the study (unless treatments assigned at randomisation were changed for the extension period). The comparative effect of denosumab and other treatments for osteoporosis was expressed as relative risk (RR) for fractures or adverse events, Peto odds ratio (OR) for withdrawals due to adverse events and death and mean difference for percent BMD change from baseline. A 95 % confidence interval (95 % CI) was constructed for each measure of effect. The Mantel-Haenszel method was used for the meta-analysis on fractures [11] and the generic inverse variance method for those on adverse events and percent BMD changes from baseline. To account for between-study heterogeneity, random effects methods were used except when a Peto OR was computed. Results for hip and vertebral fractures were summarised qualitatively as too few events were reported for meta-analyses. Heterogeneity was quantified using the I2 statistic and the thresholds proposed in the Cochrane Handbook for Systematic Reviews of Interventions (0–40 %: low, 30–60 %: moderate, 50–90 %: substantial, 75–100 %: considerable heterogeneity) [9]. Subgroup analyses were planned a priori and conducted for the following variables: gender, treatment for osteoporosis before the study, BMD level at the beginning of the study (lower vs higher), administration of a cointervention, comparison treatment (alendronate vs other treatments, bisphosphonates vs other treatments), treatment frequency and dosage, overall risk of bias and funding source. A study was assigned to the lower BMD T-score subgroup if its population included only participants with a BMD T-score lower than −2, but also some with a BMD T-score lower than −3. In sensitivity analyses, meta-analyses on all outcomes were performed using 12-month follow-up data. For each meta-analysis conducted, a funnel plot was constructed to assess risk of publication bias.
Results
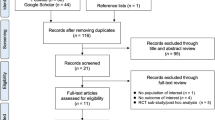
Among the 1764 articles identified using our search strategy, 13 satisfied the eligibility criteria (Fig. 1). These articles refer to 9 different studies which included a total of 4890 postmenopausal women (Table 1). Denosumab was administered subcutaneously at a dosage of 60 mg every 6 months in eight studies [12–21]. In one study [22–24], 6, 14, 30, 60, 100 or 210 mg of denosumab was administered every 3 or 6 months. In the meta-analyses, data from this study were first used by regrouping all participants who received denosumab, regardless of dosage and frequency. In a sensitivity analysis on the comparative effect of 60 mg of denosumab every 6 months, we then only used data from the appropriate randomisation group when available. The effect of denosumab was compared to alendronate in five studies [12–16, 22–24] and to zoledronic acid, risedronate, ibandronate or teriparatide in four individual studies [17–21]. Follow-up duration varied from 12 to 24 months.
A high, unclear and low overall risk of bias was respectively adjudicated to five [15–20, 22–24], one [21] and three [12–14] studies. In all studies, adverse events were collected in at least 80 % of randomised subjects (Appendix B). The authors of only five studies [12, 13, 17, 20, 21] specified that at least 80 % of randomised patients were included in the BMD analyses. Participants and assessors were blinded to treatments in three studies [12–14]. In only one study [18, 19], AMGEN had no role in the study design, data collection, data analysis, data interpretation or writing of the report. All studies were funded by AMGEN.
Comparative effect of denosumab on fracture risk
Seven studies [12, 13, 15–17, 20–24] in which the number of fractures was specified for each treatment group were included in the meta-analysis on fracture risk. In all studies, the effect of denosumab was compared to a specific bisphosphonate. No statistically significant difference was detected between the fracture risk of participants who received denosumab and those who received a bisphosphonate (n = 4602, RR[95 % CI] = 1.15 [0.84–1.58], I2 = 0 %, Fig. 2). The funnel plot did not suggest the presence of a publication bias (Appendix C). No significant difference was detected in any of the sensitivity or subgroup analyses (Table 1 of Appendix D).
Only three studies reported the number of participants who suffered a hip or vertebral fracture [13, 15–17]. In two studies [13, 15, 16], no participants in the denosumab or alendronate group suffered a fracture at these sites. In another study [17], 1/410 and 2/410 participants randomised to ibandronate suffered a hip and vertebral fracture, respectively, and 2/411 randomised to denosumab suffered a vertebral fracture.
Comparative effect of denosumab on adverse events
Among the nine studies included in this review, eight [12–17, 20–24] specified the total number of adverse events reported in each randomisation group. All studies compared the effect of denosumab to a bisphosphonate. Based on data from these studies, the risk of adverse events was not significantly different between participants who received denosumab and those who received a bisphosphonate (n = 4766, RR[95 % CI] = 0.99 [0.96–1.02], I2 = 0 %, Fig. 3). The absence of symmetry in the funnel plot suggests that smaller studies favouring denosumab instead of other treatments for osteoporosis may not have been published (Appendix E). Since all studies included in this review were funded by AMGEN, the manufacturer of denosumab, the presence of such a publication bias is unlikely. We did not detect a significant difference in the risk of adverse events between the groups in any of the subgroups analysed (Table 2 of Appendix D).
All studies [12–24] specified the number of withdrawals due to adverse events and could thus be included in the meta-analysis on this outcome. The risk of withdrawals due to adverse events was lower in participants treated with denosumab than in those randomised to another treatment, but the difference did not reach statistical significance (n = 4887, OR[95 % CI] = 0.68 [0.45–1.04], I2 = 57 %, Fig. 4). Results were similar when denosumab was compared to bisphosphonates (n = 4545, OR[95 % CI] = 0.70 [0.46–1.08], Table 3 of Appendix D). In the studies not exclusively including subjects who had previously received a treatment for osteoporosis [12, 14–16, 18, 19, 22–24], the risk of withdrawals due to adverse events was significantly lower in participants treated with denosumab (n = 2040, OR[95 % CI] = 0.48 [0.25–0.92], I2 = 0 %). In studies including participants with a lower BMD T-score [12, 13, 15–17, 21], treatment with denosumab was also associated with a lower risk of withdrawals due to adverse events (n = 3416, OR[95 % CI] = 0.49 [0.29–0.83], I2 = 25 %).
In the six studies included in the meta-analysis on death [12, 13, 17, 20–24], the comparator treatment was a bisphosphonate. The risk of death was not statistically different between denosumab and bisphosphonate groups (n = 4360, OR[95 % CI] = 0.58 [0.12–2.71], I2 = 0 %, Fig. 1 of Appendix F). No significant association was detected in any of the subgroups examined (Table 4 of Appendix D).
Comparative effect of denosumab on changes in bone mineral density
Among the nine studies included in this review, eight [12, 13, 15–24] evaluated the comparative effect of denosumab on BMD at the total hip, lumbar spine, femoral neck or one-third radius. The percent changes in BMD measured at all of these sites were statistically higher in participants randomised to denosumab than in other treatment groups (n, mean difference [95 % CI]; total hip 4434, 1.06 [0.86–1.25], lumbar spine 4415, 1.46 [0.97–1.95], femoral neck 4153, 1.06 [0.81–1.30], one-third radius 2571, 1.12 [0.47–1.78], Fig. 5 and Figs. 2 to 4 of Appendix F). Results of the meta-analyses comparing the effect of denosumab to bisphosphonates were similar (mean difference [95 % CI]; total hip 1.05 [0.85–1.26], lumbar spine 1.55 [1.09–2.02], femoral neck 1.06 [0.79–1.32], one-third radius 0.83 [0.34–1.31], Tables 5–8 of Appendix D). At the total hip and femoral neck, the difference in treatment effects was significant in all subgroups investigated (Tables 5 and 7 of Appendix D). While the change in BMD at the lumbar spine and one-third radius was almost always higher in the denosumab group, there was considerable heterogeneity in the meta-analyses on these outcomes (I2 = 73 and 76 %, respectively). When comparing the effect of denosumab to alendronate only, denosumab was still more effective in increasing BMD at the lumbar spine and heterogeneity was no longer present (I2 = 0 %) (Table 6 of Appendix D).
Discussion
In this meta-analysis, no significant difference between denosumab and bisphosphonates was detected in total adverse events, death or fracture occurrence over a period of 12 to 24 months. Withdrawals due to adverse events tended to be less frequent in participants treated with denosumab, but the difference did not reach statistical significance. Denosumab was slightly more effective than other treatments were, including bisphosphonates, in increasing areal BMD.
When compared to other treatments for osteoporosis, mostly bisphosphonates, denosumab provided an additional approximate gain of 1 % in BMD over 12 to 24 months. The superiority of denosumab over bisphosphonates in increasing BMD could be explained by a greater antiresorptive effect. While bisphosphonate inhibition of osteoclasts requires binding to bone mineral, denosumab acts directly by binding to RANKL. When bound to RANKL, denosumab distinctively inhibits osteoclast formation, function and survival [25].
While denosumab showed a superior effect on BMD, we did not observe a higher efficacy than bisphosphonates in reducing fracture risk. We could presume that the gain in BMD over a short period of time was not large enough to reduce fracture incidence. The differential effect of denosumab on fracture risk may only become apparent after a longer period of treatment than that used in included studies.
In a network meta-analysis on the efficacy of different drugs to prevent fragility fractures [26], denosumab, teriparatide, alendronate, zoledronate and risedronate were all more effective than the placebo was in reducing the risk of non-vertebral fractures. No significant differences between treatment effects were observed which is consistent with our findings. In a Bayesian analysis based on indirect comparisons of treatments, teriparatide had the highest probability of being ranked as the most effective treatment to reduce fracture incidence. In our review, we only included head-to-head trials comparing denosumab to other treatments for osteoporosis. Since we identified only one small study which compared the effect of denosumab to teriparatide, we could not draw conclusions on this specific treatment comparison.
In another meta-analysis on the comparative efficacy and safety of denosumab and alendronate [27], Lin et al. did not detect a difference for safety or fracture risk over a 1-year follow-up period. They also concluded that denosumab was more effective in increasing BMD. Although their conclusions on the comparative effect of denosumab on BMD are similar to ours, our results are slightly different. We noted that they inadvertently used standard errors instead of the standard deviation in their meta-analysis on the change in BMD, which created narrower confidence intervals.
Recent reviews [28, 29] have shown that, in a clinical setting, persistence and compliance with denosumab is significantly better than with oral bisphosphonates. In the present review, only randomised controlled trials were included. In such studies, patients are closely followed and persistence and compliance tend to be higher than in a real-world setting. In the real-world clinical practice, denosumab may thus be more effective than what is shown in this review.
A recent literature review [30] on the cost-effectiveness of denosumab complements the results of our review. The authors showed that denosumab was more cost-effective than most other treatments are for osteoporosis, including oral medication for which the average wholesale price is considerably lower [31]. As they suggested, the higher cost-effectiveness of denosumab compared to many other medications for osteoporosis could, amongst other things, be attributable to frequency of administration. Since denosumab is administered once every 6 months rather than every week, patients’ adherence may be increased, thus increasing its effectiveness to prevent fractures in a clinical setting.
The main limitation of our systematic review is that included studies have a high risk of bias and all studies were funded by AMGEN which was actively involved in the study design and/or conduct of all but one study. Another important limitation is that none of the studies were designed to evaluate the comparative efficacy of denosumab in decreasing fracture risk. The change in BMD and level of bone turnovers were the main outcomes and fractures were reported as adverse events without a specific adjudication process. None of the individual studies were powered to detect a significant difference in fracture risk between groups. While performing a meta-analysis is a way to increase statistical power, the number of individuals included in meta-analyses on fracture risk may still not be sufficient [32]. Since very few deaths were reported in each study, results of the meta-analysis on death are imprecise. None of the included studies were performed in men; thus, the results of this meta-analysis cannot be generalised to males. This review includes only studies that were conducted over a follow-up period of 12 or 24 months. Consequently, the comparative effect of prolonged use of denosumab could not be studied. Despite these limitations, the results of our review are based on an exhaustive literature search and rigorous methodology. The probability of publication bias was judged low and only head-to-head randomised control trials were included.
The results of this meta-analysis do not offer evidence of the differential safety of denosumab compared to bisphosphonates in treating individuals at risk for osteoporosis. While denosumab was significantly more effective in increasing BMD, its use did not lead to a significant reduction in fracture risk. In real-world clinical practice, denosumab may, however, be more effective given its higher persistence and compliance. Until more studies are conducted, this research suggests that denosumab could be a good alternative to other antifracture medications. When choosing a patient’s medication, patient particularities (tolerance, adherence, comorbidities, etc.) should be considered. More studies on the comparative efficacy and safety of denosumab should be performed, particularly in men, on longer follow-up periods and using fracture as the primary outcome.
References
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285(6):785–795
Kanis JA (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 4(6):368–381
WHO Scientific Group (2003) Prevention and management of osteoporosis. WHO technical report series. World Health Organization, Geneva
Binkley NC, Schmeer P, Wasnich RD, Lenchik L (2002) What are the criteria by which a densitometric diagnosis of osteoporosis can be made in males and non-Caucasians? J Clin Densitom 5(Suppl):S19–S27
U.S. Food and Drug Administration Drug Details. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails. Accessed March 1st 2014
AMGEN Prolia (denosumab) [Prescribing Information]. http://pi.amgen.com/united_states/prolia/prolia_pi.pdf. Accessed March 1st 2014
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361(8):756–765. doi:10.1056/NEJMoa0809493
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. doi:10.1136/bmj.b2535
Higgins JPT, Green S (editors) (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]
Wong SS, Wilczynski NL, Haynes RB (2006) Comparison of top-performing search strategies for detecting clinically sound treatment studies and systematic reviews in MEDLINE and EMBASE. J Med Libr Assoc 94(4):451–455
Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A (2007) Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med 26(1):53–77. doi:10.1002/sim.2528
Brown JP, Prince RL, Deal C, Recker RR, Kiel DP, de Gregorio LH, Hadji P, Hofbauer LC, Alvaro-Gracia JM, Wang H, Austin M, Wagman RB, Newmark R, Libanati C, San Martin J, Bone HG (2009) Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 24(1):153–161. doi:10.1359/jbmr.080901
Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, Man HS, San Martin J, Bone HG (2010) Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 25(1):72–81. doi:10.1359/jbmr.090716
Seeman E, Delmas PD, Hanley DA, Sellmeyer D, Cheung AM, Shane E, Kearns A, Thomas T, Boyd SK, Boutroy S, Bogado C, Majumdar S, Fan M, Libanati C, Zanchetta J (2010) Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res 25(8):1886–1894. doi:10.1002/jbmr.81
Kendler DL, McClung MR, Freemantle N, Lillestol M, Moffett AH, Borenstein J, Satram-Hoang S, Yang YC, Kaur P, Macarios D, Siddhanti S (2011) Adherence, preference, and satisfaction of postmenopausal women taking denosumab or alendronate. Osteoporos Int 22(6):1725–1735. doi:10.1007/s00198-010-1378-z
Freemantle N, Satram-Hoang S, Tang ET, Kaur P, Macarios D, Siddhanti S, Borenstein J, Kendler DL (2012) Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 23(1):317–326. doi:10.1007/s00198-011-1780-1
Recknor C, Czerwinski E, Bone HG, Bonnick SL, Binkley N, Palacios S, Moffett A, Siddhanti S, Ferreira I, Ghelani P, Wagman RB, Hall JW, Bolognese MA, Benhamou CL (2013) Denosumab compared with ibandronate in postmenopausal women previously treated with bisphosphonate therapy: a randomized open-label trial. Obstet Gynecol 121(6):1291–1299. doi:10.1097/AOG.0b013e318291718c
Tsai JN, Uihlein AV, Lee H, Kumbhani R, Siwila-Sackman E, McKay EA, Burnett-Bowie SA, Neer RM, Leder BZ (2013) Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet 382(9886):50–56. doi:10.1016/s0140-6736(13)60856-9
Leder BZ, Tsai JN, Uihlein AV, Burnett-Bowie SA, Zhu Y, Foley K, Lee H, Neer RM (2014) Two years of denosumab and teriparatide administration in postmenopausal women with osteoporosis (the DATA extension study): a randomized controlled trial. J Clin Endocrinol Metab:jc20134440. doi:10.1210/jc.2013-4440
Roux C, Hofbauer LC, Ho PR, Wark JD, Zillikens MC, Fahrleitner-Pammer A, Hawkins F, Micaelo M, Minisola S, Papaioannou N, Stone M, Ferreira I, Siddhanti S, Wagman RB, Brown JP (2014) Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open-label study. Bone 58:48–54. doi:10.1016/j.bone.2013.10.006
Miller PD, Pannacciulli N, Brown JP, Czerwinski E, Nedergaard BS, Bolognese MA, Malouf J, Bone HG, Reginster JY, Singer A, Wang C, Wagman RB, Cummings SR (2015) Denosumab compared with zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates: efficacy and safety results from a randomized fouble-blind study. J Bone Miner Res 30(S1):S1. doi:10.1002/jbmr.2763
McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, Peacock M, Miller PD, Lederman SN, Chesnut CH, Lain D, Kivitz AJ, Holloway DL, Zhang C, Peterson MC, Bekker PJ (2006) Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354(8):821–831. doi:10.1056/NEJMoa044459
Lewiecki EM, Miller PD, McClung MR, Cohen SB, Bolognese MA, Liu Y, Wang A, Siddhanti S, Fitzpatrick LA (2007) Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res 22(12):1832–1841. doi:10.1359/jbmr.070809
Beck TJ, Lewiecki EM, Miller PD, Felsenberg D, Liu Y, Ding B, Libanati C (2008) Effects of denosumab on the geometry of the proximal femur in postmenopausal women in comparison with alendronate. J Clin Densitom 11(3):351–359. doi:10.1016/j.jocd.2008.04.001
Baron R, Ferrari S, Russell RG (2011) Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 48(4):677–692. doi:10.1016/j.bone.2010.11.020
Murad MH, Drake MT, Mullan RJ, Mauck KF, Stuart LM, Lane MA, Abu Elnour NO, Erwin PJ, Hazem A, Puhan MA, Li T, Montori VM (2012) Clinical review. Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J Clin Endocrinol Metab 97(6):1871–1880. doi:10.1210/jc.2011-3060
Lin T, Wang C, Cai XZ, Zhao X, Shi MM, Ying ZM, Yuan FZ, Guo C, Yan SG (2012) Comparison of clinical efficacy and safety between denosumab and alendronate in postmenopausal women with osteoporosis: a meta-analysis. Int J Clin Pract 66(4):399–408. doi:10.1111/j.1742-1241.2011.02806.x
Karlsson L, Lundkvist J, Psachoulia E, Intorcia M, Strom O (2015) Persistence with denosumab and persistence with oral bisphosphonates for the treatment of postmenopausal osteoporosis: a retrospective, observational study, and a meta-analysis. Osteoporos Int 26(10):2401–2411. doi:10.1007/s00198-015-3253-4
Institut national d’excellence en santé et en services sociaux (INESSS) (2014) Portrait de l’usage des bisphosphonates et du dénosumab chez les personnes de 50 ans ou plus souffrant d’ostéoporose couvertes par le régime public d’assurance médicaments. INESSS, Québec
Hiligsmann M, Boonen A, Dirksen CD, Ben Sedrine W, Reginster JY (2013) Cost-effectiveness of denosumab in the treatment of postmenopausal osteoporotic women. Expert Rev Pharmacoecon Outcomes Res 13(1):19–28. doi:10.1586/erp.12.76
Green W (2010) Denosumab (Prolia) Injection: a new approach to the treatment of women with postmenopausal osteoporosis. Pharm Ther 35(10):553–559
Kanis JA, Alexandre JM, Bone HG, Abadie E, Brasseur D, Chassany O, Durrleman S, Lekkerkerker JF, Caulin F (2003) Study design in osteoporosis: a European perspective. J Bone Miner Res 18(6):1133–1138. doi:10.1359/jbmr.2003.18.6.1133
Acknowledgments
We gratefully acknowledge the contributions of Dr. Alexis F. Turgeon and Amélie Boutin for their methodological assistance. We also acknowledge the contribution of the administrative assistant Julie Parrot.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
C Beaudoin received a scholarship from the CHU de Québec.
Conflicts of interest
C Beaudoin, S Jean and L Moore have no conflict of interest to disclose.
L Bessette received remuneration from Amgen, Eli Lilly, Novartis, and Merck; consultant/advisory role to Amgen, UCB, Pfizer, Abbvie, and Hoffmann-La Roche; and funds from Abbott, Amgen, Bristol-Myers Squibb, Eli Lilly, Merck, Novartis, Pfizer, Roche, Sanofiaventis, Servier, Warner Chilcott, and Takeda.
L-G Ste-Marie received research grants from Amgen and Eli Lilly; educational grants from Amgen, Eli Lilly, Genzyme, and Novartis; and board membership from Amgen, Eli Lilly, and Merck.
J P Brown received research grants from Amgen and Eli Lilly; board membership for Amgen, Eli Lilly, and Merck; consulting and speaking fees from Amgen and Eli Lilly.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 374 kb)
Rights and permissions
About this article
Cite this article
Beaudoin, C., Jean, S., Bessette, L. et al. Denosumab compared to other treatments to prevent or treat osteoporosis in individuals at risk of fracture: a systematic review and meta-analysis. Osteoporos Int 27, 2835–2844 (2016). https://doi.org/10.1007/s00198-016-3607-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3607-6