Abstract
Summary
Whether infant vitamin D supplementation may have long-term bone benefits is unclear. In this study, breastfed infants who received vitamin dosages greater than 400 IU/day did not have higher bone mineralization at 3 years. This study provides important data to inform pediatric public health recommendations for vitamin D.
Introduction
North American health agencies recommend breastfed infants should be supplemented with 400 IU of vitamin D/day to support bone health. Few studies examined the long-term benefits of early life vitamin D supplementation on bone mineralization. The objective of this study was to determine if a dose-response relationship exists between infant vitamin D supplementation, vitamin D status, and bone outcomes at 3 years of age.
Methods
This was a double-blind randomized trial of 132, 1-month-old healthy, breastfed infants from Montréal, Canada, between 2007 and 2010. In this longitudinal analysis, 87 infants (66 %) returned for follow-up at 3 years of age, between 2010 and 2013. At 1 month of age, participants were randomly assigned to receive oral cholecalciferol (vitamin D3) supplements of 400, 800, 1200, or 1600 IU/day until 12 months of age. Lumbar spine vertebrae 1–4 (LS) bone mineral density (BMD), LS and whole body bone mineral content (BMC), and mineral accretion were measured by dual-energy x-ray absorptiometry at 3 years.
Results
At follow-up, the treatment groups were similar in terms of diet, sun exposure, and demographics. There were no significant differences among the groups in LS or whole body BMC, BMD, or accretion. Although, 25(OH)D concentrations were not different among the groups, higher doses (1200 and 1600 IU/day) achieved higher 25(OH)D area under the curve from 1 to 36 months vs. 400 IU/day.
Conclusions
This is the first longitudinal follow-up of an infant vitamin D dose-response study which examines bone mineralization at 3 years of age. Dosages higher than 400 IU/day do not appear to provide additional benefits to the bone at follow-up. Larger studies with more ethnically diverse groups are needed to confirm these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The foremost functions of vitamin D are the regulation of calcium homeostasis and bone mineral metabolism [1], both of which are of particular importance to growing children. However, the value of circulating 25-hydroxyvitamin D (25(OH)D) concentrations for optimal health is not clear [2]. Health guidelines recommend a healthy range for 25(OH)D between 50 and 125 nmol/L (20–50 ng/ml) and a standard-of-care supplemental vitamin D intake of 400 IU/day for infants until provided through the diet [1, 3, 4]. To date, there have been few infant trials examining high dose vitamin D supplementation [5–7] and no study establishing long-term benefits on bone health.
The most accelerated rates of growth and bone mineral accretion occur during infancy [8]; maximizing accretion during early life has been suggested to provide later benefits to the bone [9, 10]. Vitamin D status positively associates with bone mass in infants and children [11–14] but some infant trials show no relationship [6, 15, 16] suggesting benefits may not readily appear. Specifically, positive relationships exist for vitamin D exposure in utero [11] and in infancy [17] with later bone mass in childhood. Prepubertal girls with moderate vitamin D status had significantly higher cortical radial and tibia volumetric bone mineral density (BMD) as well as lower bone resorption and parathyroid hormone (PTH) than those with deficiency (≤25 nmol/L 25(OH)D) [18]. In a cross sectional analysis of preschool age children, we observed that whole body bone mineral content (BMC) and BMD were positively linked to having 25(OH)D over 75 nmol/L [19]. However, there continues to be limited data regarding the impact of vitamin D status on bone mineral accretion in early life, requiring longitudinal follow-up.
Recently, we published a vitamin D dose-response study (NCT00381914) of daily vitamin D3 supplementation ranging from 400 to 1600 IU in breastfed infants from 1 to 12 months of age. As previously reported [5], all dosages supported 25(OH)D3 concentrations above 50 nmol/L after 2 months of supplementation; however, no significant benefits to bone mineral accretion were observed among the groups by 1 year of age. In the present study, which is a longitudinal follow-up from our study published in 2013 [5], we tested the possibility that dose-response effects of vitamin D given in infancy would be observed in bone health later in childhood. As growth in infancy sets the foundation for subsequent skeletal growth [20] and in agreement with previous studies conducted in prepubertal children [14, 17], we hypothesized that in the context of sustained healthy vitamin D status, bone health targets achieved in infancy would continue to 3 years of age. Additionally, the role of vitamin D epimers and metabolites in childhood is not well understood, and thus, secondary outcomes of this study included exploring vitamin D metabolism at 3 years.
Materials and methods
The design and main outcomes for the original study are previously described in detail [5]. Briefly, 132 healthy, term, breastfed (consuming ≥80 % of total milk volume) infants were enrolled between 2007 and 2010 from greater Montréal (Québec, Canada), located at 45°N. At 1 month of age (baseline), infants were randomized to receive one of four dosages of oral cholecalciferol (400, 800, 1200, or 1600 IU/day of vitamin D3) and were monitored at study visits conducted at 3, 6, 9, and 12 months of age. The 1600 IU/day group was discontinued early in the study due to plasma 25(OH)D3 concentrations of 250 nmol/L or greater by 3 months of age based on an enzyme immunoassay (see [5] for stopping rules). On average, participants were supplemented with the study vitamin D for 11 months, as reported compliance with supplementation ranged between 84 and 90 % over the course of the trial. At the final trial visit, completed at 12 months of age, all parents regardless of the infant treatment group were advised about dietary sources of vitamin D and encouraged to provide a commercially available supplement of 400 IU/day (based on IOM recommendation prior to 2011), if they were unable to get this amount through their diet. The present observational follow-up was conducted when the children were 36 months (3 years) of age, between 2010 and 2013. McGill University and George Mason University institutional review boards provided ethical approval. Parents self-identified race/ethnicity, using Canadian Census criteria, and demographics including education and income were collected.
Anthropometry
Height was measured (nearest mm) using a stadiometer (Seca 213, Seca Medical Scales and Measuring Systems) and weight using a calibrated scale (Detecto) with the child wearing light clothing and no shoes. BMI was calculated as kg/m2, and weight-for-age (WAZ), height-for-age (HAZ), and BMI-for-age (BAZ) Z-scores were derived based on the World Health Organization 2007 growth standards (WHO AnthroPlus) [21].
Laboratory analysis
A non-fasted capillary blood sample (lithium heparin) in addition to a spot urine sample was collected. To assess calcium homeostasis, blood-ionized calcium (ABL 725 series blood gas analyzer; Radiometer America), and plasma total calcium, phosphorus, alkaline phosphatase, and urinary calcium:creatinine were measured (Beckman Coulter DXC600) immediately with intra-assay coefficient of variation (CV) of <5 %. Additional plasma was stored at −80 °C for batch analysis. Plasma intact PTH was measured using an ELISA (Immutopics International) sensitive to 12 pg/mL (12 ng/L) and CV <8 %. Plasma C-terminal telopeptide of type I collagen (CTX), a biomarker of bone turnover, was measured using CrossLaps® ELISA (Immunodiagnostic Systems Ltd) sensitive to 0.020 ng/mL and CV 9.4 %. We have previously noted high proportions of both 3-epimer-25(OH)D (3-epi-25(OH)D3) and 24,25-dihydroxyvitamin D (24,25(OH)2D3) in relation to plasma 25(OH)D3 [22]; thus, in order to further explore the relationship between vitamin D metabolism and bone accretion, plasma 3-epi-25(OH)D3, 24,25(OH)2D3 and 25(OH)D3 were quantified by Warnex Bioanalytical Services using liquid-chromatography tandem mass spectrometry (LC-MS/MS) [23]. See [5, 22] for method description. The intra-assay CVs were <15 % for all vitamin D metabolites; the laboratory was certified by the Vitamin D External Quality Assessment Scheme. The measured 25(OH)D3 concentrations of the National Institute of Standards and Technology (NIST) standard reference materials (SRM 968e) [24] were within 7.0 and 2.5 % of the certified values for levels 2 and 3, respectively. The ability to achieve plasma 25(OH)D concentrations of ≥50 and ≥75 nmol/L was explored as both are considered health targets [3, 4, 25].
Dietary data
Dietary intake was measured using three 24-h recalls (one in person, two by telephone), and nutrient intake was generated using Nutritionist Pro software version 4.7.0 (Axxya Systems LLC) using the 2010b Canadian Nutrient File database (Health Canada). The proportion of children achieving the estimated average requirement (EAR; 400 IU/day) and recommended dietary allowance (RDA; 600 IU/day) was assessed. In addition, a validated food frequency questionnaire (FFQ) for preschool children, completed by the primary caregiver, was used to capture calcium and vitamin D intake over the past month [26]. Total dietary and supplemental vitamin D intake was categorized into tertiles. Cows’ milk and margarine are mandated to be fortified with vitamin D in Canada [27], as natural dietary sources of vitamin D are limited. Fortified cow’s milk contributes 66–72 % of total vitamin D intake to Canadian children’s diet [28].
Sun exposure
Questionnaires were used to assess sun exposure, winter travel, and use of sunscreen for the 2 years prior to the follow-up visit. Sun exposure was collected retrospectively as a percentage of body surface area (BSA) exposed, frequency of sunscreen use, and total hours spent in direct sunlight per day. Sun index was calculated for each child by multiplying the percent BSA exposed by the time spent outside (minutes per day) [29]. This index does not consider the use of sun block. Season of visit was defined using the equinox and solstice dates [30].
Skin pigmentation
Skin pigmentation was measured on the constitutive upper underarm and facultative forehead, forearm, and outer lower leg using a portable computerized spectrophotometer (CM-600D, Konica Minolta). Based on the Commission Internationale de l’Eclairage colorimetry system (L*a*b*), the individual typological angle (ITA) {ITA° = [arc tangent (L* − 50) / b*)] 180/3.14159} of the upper underarm site was calculated [31]. Infants were classified into five skin phototypes as follows: dark (≤10°), olive (10–28°), medium (28–41°), fair (41–55°), and very fair (>55°).
Bone parameters
Bone area, bone mineral content (BMC), and areal bone mineral density (aBMD) of the whole body and L1 to L4 vertebrae were measured using dual-energy x-ray absorptiometry (DXA; QDR 4500A, APEX software version 13.2.3 or 13.3.3, Hologic Inc., Waltham, MA). Children were scanned according to International Society for Clinical Densitometry (ISCD) guidelines [32]; whole body was scanned using infant whole body software between 0–12 months and adult whole body at 36 months, whereas lumbar spine scans were captured using anterior-posterior software (express mode at 36 months). Values are expressed as absolute BMC, aBMD, Z-scores (reference: Pediatric, Hologic 2005 [33]), % accretion (i.e., Δ BMC from 36–1 month/BMC at 1 month × 100 %) as well as accretion/year (i.e., Δ BMC from 36–1 month/(days in period/365 days/year)). Whole body BMC was further corrected for weight, height, and lean mass as suggested by the ISCD [32]. The CV was 1 % for BMC and 0.3 % for BMD using a spine phantom (Hologic phantom No. 14774).
Statistical methods
Baseline differences among the groups were tested using analysis of variance (ANOVA) and Χ 2. The mean area under the curve (AUC) for 25(OH)D concentrations across all time points was calculated based on the trapezoidal method. The effects of vitamin D treatment on plasma vitamin D metabolite and epimer concentrations, AUC, anthropometry, biomarkers of bone metabolism, and bone mineral accretion were explored and tested using mixed-model ANOVA analyzed as intent-to-treat. Participants with missing data are not dropped using this procedure. Post hoc tests (estimates statements) were used to test for differences between the treatment groups and Bonferroni adjustment for multiple comparisons applied. Data analysis was conducted both including and excluding the 1600 IU/day group (n = 11) with similar results; therefore, all the groups were included in the final analysis. Using an alpha level of 0.05 for a two-tailed test and 90 % power, a sample size of 25 subjects/group would detect a 5 % difference in whole body BMD (0.56 to 0.59 g/cm2). Statistical significance for all the tests was set at p < 0.05 after adjustment for multiple comparisons where applicable. Values reported are in mean [95 % CI] or number (%).
Results
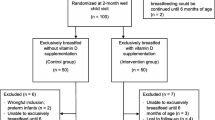
From the original 132 participants in the trial, 66 % (49 boys and 38 girls) returned for the 3-year follow-up (average 36.7 months). This represented 87 % of those who completed the original study (85 of 98) and 2 drop-outs, which returned for follow-up (Online Resource Fig. 1). All children were healthy, and four (5 %) children were categorized as >2 SD based on BAZ. Except for age being greater in the 1600 IU group, there were no differences among the groups in demographic, growth, nutrition, and sun exposure factors at 3 years (Table 1). There were no differences in the treatment group allocation among returners vs. non-returners; however, mothers of returners were older (p = 0.05), typically white (p = 0.01) and differed by season of sampling (p = 0.04) than those of non-returners. There were no significant differences in 25(OH)D concentrations or status at 3 years between returners and non-returners (Online Resource Table 1).
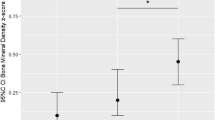
No differences among the vitamin D treatment groups were noted for BMC, BMD, or BMD Z-score of the LS or whole body (Table 2). In line with previous results [19], sex did not affect bone outcomes; thus, data are presented combined. No significant difference in lumbar spine or whole body body mineral accretion among the groups was noted. There were no effects of treatment or vitamin D status at 3 years on biomarkers related to the bone including ionized calcium (mean 5.16, 95 % CI 5.08–5.20 mg/dL), plasma CTX (1.13, 1.06–1.20 ng/mL), or PTH (34.4, 31.8–37.1 pg/mL) at 3 years.
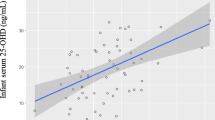
At 3 years, the mean plasma 25(OH)D3 for all the children was 73.6 nmol/L, 25(OH)D3:24,25(OH)2D ratio was 8:1, and 25(OH)D3:3-epi-25(OH)2D3 was 20:1. Plasma 25(OH)D3, 24,25(OH)2D3, and 3-epimer-25(OH)D3 were not different among the groups (Fig. 1a). Significant differences were noted between the 1200 and 1600 IU/day vs. 400 IU/day in 25(OH)D AUC from 1 to 36 months (p < 0.01) (Fig. 1b). Of all the children at 3 years, none were below 30 nmol/L, 1 % (n = 1 of 87) fell below 40 nmol/L, 10 % (n = 9) below 50 nmol/L, and 62 % (n = 54) below 75 nmol/L of 25(OH)D. This reflects the following changes in 25(OH)D from 1 month of age: 6 % (n = 4 of 65) below 30 nmol/L, 17 % (n = 11) below 40 nmol/L, 29 % (n = 19) below 50 nmol/L, and 72 % (n = 47) below 75 nmol/L of 25(OH)D. At 3 years, the proportion of children achieving the 50 or 75 nmol/L cut-off was not statistically different among the groups (Fig. 1c).
Data are mean, and bars indicate 95 % CIs. *p < 0.05 vs. 400 IU/day. a Plasma 25(OH)D3, 24,25(OH)2D3, and 3-epi-25(OH)D3 concentrations at 36 months by the treatment group. b Plasma 25(OH)D3 AUC from 1 to 36 months by the treatment group. c Percentage of children achieving at least 50 and 75 nmol/L of plasma 25(OH)D3 at 36 months by the treatment group
Discussion
This is the first follow-up study to explore bone health outcomes at 3 years of age in an infant cohort randomized to receive vitamin D supplementation between 400 and 1600 IU/day from 1 to 12 months of age. In the present follow-up of previously breastfed infants, receipt of more than 400 IU of vitamin D/day did not appear to provide additional benefits for bone mineralization at 3 years of age, thus providing support for our current infant vitamin D health policy recommendations [1, 3] in the amount of 400 IU/day. Overall, children at 3 years were all healthy, growing and consuming an adequate diet, with no differences among the groups. Macronutrient intakes were in line with the acceptable macronutrient distribution ranges [34]; dietary calcium intakes were above the RDA of 700 mg/day; however, vitamin D intake from diet alone was unable to meet the RDA of 600 IU/day in all the children.
In a previous retrospective analysis [17], girls receiving an infant vitamin D supplement of 400 IU/day over a 12-month period (range 2–48 months) had a 6 % higher BMD of the distal radius and 9 % higher femoral neck at 7–9 years, after adjustment for height, implying supplementation during infancy may have long-term bone benefits. A recent meta-analysis [35] reported vitamin D supplementation during childhood led to improvements in the bone in a sub-group of vitamin D-deficient children (25(OH)D3 < 35 nmol/L) but not in healthy children. The lack of dosage effect on bone outcomes in the present sample of children may be attributed to the robust baseline plasma 25(OH)D concentrations at 1 month of age, with an average of 56 nmol/L (95 % CI, 56–67 nmol/L) and very few (6 %) below 30 nmol/L. BMD values for this group of children were above average with BMD Z-score for the lumbar spine of 0.4 and whole body of 2.0. It is unclear whether the higher whole body Z-scores observed in the present study reflect differences in equipment models, scan modes or software versions, or population characteristics compared to the reference [33] or true differences in bone density. Discrepancies among pediatric reference data have been previously observed [36–38] with larger variation due to younger age [38].
These data add to our knowledge on the biological activity of vitamin D metabolites and epimers on pediatric bone. Although the function of 3-epi-25(OH)D3 is unknown, our study [5, 22] as well as others [39] have shown the 3-epi-25(OH)D3 to be high during the first year of life (95 % CI 19.8–22.9 nmol/L) [5] and decline by 3 years (95 % CI 2.7–4.0 nmol/L). The value of 24,25(OH)2D3 stayed fairly constant from 12 months (95 % CI 9.1–10.9 nmol/L) to 3 years (95 % CI 11.0–17.0 nmol/L). Circulating levels of 3-epi-25(OH)D3 may reflect vitamin D intake, which concurs with cell culture and animal studies [40], as 12-month-old infants in our dose-response trial were receiving between 42 and 166 IU/kg/day vs. 3-year olds ∼33 IU/kg/day of vitamin D from both diet and supplements. In addition, the high concentrations of 25(OH)D and 3-epi-25(OH)D3 observed in some infants during infancy [22] do not appear to adversely affect bone or PTH at 3 years as all the groups had similar absolute BMC and BMD.
The current sample at 3 years may not have been representative of our initial sample, although 66 % returned for follow-up, maternal characteristics differed in returners, and statistical analysis of imbalance was not performed to account for loss at follow-up. Our population studied was underrepresented in participants with darker skin pigmentation, who are known to be at higher risk of deficiency [1, 4]. A larger sample size would have allowed us to eliminate the chance of type II error. Some measures (i.e., sun exposure) may be subject to recall bias, and parental knowledge about the vitamin D dosage group may have influenced the results. Further, other technologies including peripheral quantitative computed tomography (pQCT) would allow the assessment of bone geometry in addition to BMC and BMD [41]. In order to verify our findings, it would be desirable to measure trabecular vs. cortical BMD by pQCT [6, 41] allowing detection of changes to different bone compartments. Lastly, regarding our biochemical indicators, no significant effects were noted along with bone mass, in line with a previous infant vitamin D supplementation trial [6]. Some markers may be sensitive to diurnal variation and feeding [42, 43] which were not controlled for in the study design.
In conclusion, these are the first results from an infant vitamin D supplementation trial to explore bone health effects on children at 3 years of age. This study supports our current infant vitamin D policy recommendation; however, larger studies with more ethnically diverse groups are needed to confirm these results.
References
Institute of Medicine (2011) Dietary reference intakes for calcium and vitamin D. National Academy Press, Washington, DC
Leidig-Bruckner G, Roth HJ, Bruckner T et al (2011) Are commonly recommended dosages for vitamin D supplementation too low? Vitamin D status and effects of supplementation on serum 25-hydroxyvitamin D levels—an observational study during clinical practice conditions. Osteoporos Int 22:231–240. doi:10.1007/s00198-010-1214-5
Wagner CL, Greer FR, American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition (2008) Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 122:1142–1152. doi:10.1542/peds.2008-1862
Godel JC, Canadian Paediatric Society, First Nations, Inuit and Métis Health Committe (2007) Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatr Child Health 12:583–598
Gallo S, Comeau K, Vanstone C et al (2013) Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA J Am Med Assoc 309:1785–1792. doi:10.1001/jama.2013.3404
Holmlund-Suila E, Viljakainen H, Hytinantti T et al (2012) High-dose vitamin D intervention in infants—effects on vitamin d status, calcium homeostasis, and bone strength. J Clin Endocrinol Metab 97:4139–4147. doi:10.1210/jc.2012-1575
Ziegler EE, Nelson SE, Jeter JM (2014) Vitamin D supplementation of breastfed infants: a randomized dose-response trial. Pediatr Res. doi:10.1038/pr.2014.76
Gallo S, Vanstone CA, Weiler HA (2012) Normative data for bone mass in healthy term infants from birth to 1 year of age. J Osteoporos 2012:672403. doi:10.1155/2012/672403
Cooper C, Javaid MK, Taylor P et al (2002) The fetal origins of osteoporotic fracture. Calcif Tissue Int 70:391–394. doi:10.1007/s00223-001-0044-z
Holroyd C, Harvey N, Dennison E, Cooper C (2012) Epigenetic influences in the developmental origins of osteoporosis. Osteoporos Int 23:401–410. doi:10.1007/s00198-011-1671-5
Mahon P, Harvey N, Crozier S et al (2010) Low maternal vitamin D status and fetal bone development: cohort study. J Bone Miner Res Off J Am Soc Bone Miner Res 25:14–19. doi:10.1359/jbmr.090701
Weiler H, Fitzpatrick-Wong S, Veitch R et al (2005) Vitamin D deficiency and whole-body and femur bone mass relative to weight in healthy newborns. CMAJ 172:757–761. doi:10.1503/cmaj.1040508
Viljakainen HT, Saarnio E, Hytinantti T et al (2010) Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab 95:1749–1757. doi:10.1210/jc.2009-1391
Javaid MK, Crozier SR, Harvey NC et al (2006) Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 367:36–43. doi:10.1016/S0140-6736(06)67922-1
Greer FR, Searcy JE, Levin RS et al (1982) Bone mineral content and serum 25-hydroxyvitamin D concentrations in breast-fed infants with and without supplemental vitamin D: one-year follow-up. J Pediatr 100:919–922
Roberts CC, Chan GM, Folland D et al (1981) Adequate bone mineralization in breast-fed infants. J Pediatr 99:192–196
Zamora SA, Rizzoli R, Belli DC et al (1999) Vitamin D supplementation during infancy is associated with higher bone mineral mass in prepubertal girls. J Clin Endocrinol Metab 84:4541–4544. doi:10.1210/jcem.84.12.6183
Cheng S, Tylavsky F, Kröger H et al (2003) Association of low 25-hydroxyvitamin D concentrations with elevated parathyroid hormone concentrations and low cortical bone density in early pubertal and prepubertal Finnish girls. Am J Clin Nutr 78:485–492
Hazell TJ, Pham TT, Jean-Philippe S et al (2014) Vitamin D status is associated with bone mineral density and bone mineral content in preschool-aged children. J Clin Densitom Off J Int Soc Clin Densitom. doi:10.1016/j.jocd.2014.04.121
Cooper C, Cawley M, Bhalla A et al (1995) Childhood growth, physical activity, and peak bone mass in women. J Bone Miner Res Off J Am Soc Bone Miner Res 10:940–947. doi:10.1002/jbmr.5650100615
De Onis M, Onyango AW, Borghi E et al (2007) Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85:660–667
Gallo S, Comeau K, Agellon S et al (2014) Methodological issues in assessing plasma 25-hydroxyvitamin D concentration in newborn infants. Bone 61:186–190. doi:10.1016/j.bone.2014.01.012
Singh RJ (2010) Quantitation of 25-OH-vitamin D (25OHD) using liquid tandem mass spectrometry (LC-MS-MS). Methods Mol Biol 603:509–517. doi:10.1007/978-1-60761-459-3_50
Thomas JB, Duewer DL, Mugenya IO et al (2012) Preparation and value assignment of standard reference material 968e fat-soluble vitamins, carotenoids, and cholesterol in human serum. Anal Bioanal Chem 402:749–762. doi:10.1007/s00216-011-5447-8
Holick MF, Binkley NC, Bischoff-Ferrari HA et al (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930. doi:10.1210/jc.2011-0385
El Hayek J, Pham TT, Finch S et al (2014) Validity and reproducibility of a short food frequency questionnaire in assessing calcium and vitamin D intake in Canadian preschoolers. EC Nutr 1(1):9–18
Food and Drug Regulations. CRC, c 870 (last amended on 2014 Aug. 21). http://laws-lois.justice.gc.ca/eng/regulations/C.R.C.,_c._870/. Accessed 25 Sep 2014
El Hayek J, Pham TT, Finch S et al (2013) Vitamin D status in Montréal preschoolers is satisfactory despite low vitamin D intake. J Nutr 143:154–160. doi:10.3945/jn.112.169144
Barger-Lux MJ, Heaney RP (2002) Effects of above average summer sun exposure on serum 25-hydroxyvitamin D and calcium absorption. J Clin Endocrinol Metab 87:4952–4956. doi:10.1210/jc.2002-020636
Webb AR, Kline L, Holick MF (1988) Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 67:373–378. doi:10.1210/jcem-67-2-373
Del Bino S, Sok J, Bessac E, Bernerd F (2006) Relationship between skin response to ultraviolet exposure and skin color type. Pigment Cell Res 19:606–614. doi:10.1111/j.1600-0749.2006.00338.x
Kalkwarf HJ, Abrams SA, DiMeglio LA et al (2014) Bone densitometry in infants and young children: the 2013 ISCD Pediatric Official Positions. J Clin Densitom 17:243–257. doi:10.1016/j.jocd.2014.01.002
Kelly T, Specker B, Binkley T, et al. (2006) Pediatric BMD reference database for US white children. BONE, p Suppl1:S30
Institute of Medicine (2005) Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). National Academy Press, Washington, DC
Winzenberg T, Powell S, Shaw KA, Jones G (2011) Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ 342:c7254
Ma J, Siminoski K, Alos N et al (2015) The choice of normative pediatric reference database changes spine bone mineral density Z-scores but not the relationship between bone mineral density and prevalent vertebral fractures. J Clin Endocrinol Metab 100:1018–1027. doi:10.1210/jc.2014-3096
Leonard MB, Propert KJ, Zemel BS et al (1999) Discrepancies in pediatric bone mineral density reference data: potential for misdiagnosis of osteopenia. J Pediatr 135:182–188
Noon E, Singh S, Cuzick J et al (2010) Significant differences in UK and US female bone density reference ranges. Osteoporos Int 21:1871–1880. doi:10.1007/s00198-009-1153-1
Singh RJ, Taylor RL, Reddy GS, Grebe SKG (2006) C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab 91:3055–3061. doi:10.1210/jc.2006-0710
Kamao M, Tatematsu S, Hatakeyama S et al (2004) C-3 epimerization of vitamin D3 metabolites and further metabolism of C-3 epimers: 25-hydroxyvitamin D3 is metabolized to 3-epi-25-hydroxyvitamin D3 and subsequently metabolized through C-1alpha or C-24 hydroxylation. J Biol Chem 279:15897–15907. doi:10.1074/jbc.M311473200
Zemel B, Bass S, Binkley T et al (2008) Peripheral quantitative computed tomography in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom 11:59–74. doi:10.1016/j.jocd.2007.12.006
Wu T-C, Huang I-F, Chen Y-C et al (2011) Differences in serum biochemistry between breast-fed and formula-fed infants. J Chin Med Assoc 74:511–515. doi:10.1016/j.jcma.2011.09.007
Seibel M (2005) Biochemical markers of bone turnover part I: biochemistry and variability. Clin Biochem Rev 26:97–122
Acknowledgments
The authors would like to thank Dr. Ali Khamessan, PhD Europharm International Canada Inc., for the design of the study product. We thank McGill University graduate student Sade Hayes RD, MSc for the help with study measurements and analysis of the dietary data. Finally, we would like to thank all the families who agreed to participate in the original and current follow-up study and the Mary Emily Clinical Nutrition Research Unit of the School of Dietetics and Human Nutrition.
Research funding was provided by the Canadian Institutes of Health Research and Nutricia Research Foundation, Europharm International Canada Inc. provided in-kind support of supplements. The sponsors were not involved in the design, conduct of the study, collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. Dr. Hope A. Weiler, RD PhD is a Canada Research Chair with infrastructure funding from the Canadian Foundation for Innovation. Drs. Sina Gallo and Hope Weiler had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
McGill University and George Mason University institutional review boards provided ethical approval for the study.
Conflicts of interest
Sina Gallo, Tom Hazell, Catherine A. Vanstone, Sherry Agellon, Glenville Jones, Mary L’Abbé, and Celia Rodd declare that they have no conflict of interest. Hope Weiler is a Canada Research Chair with infrastructure funding from the Canadian Foundation for Innovation.
Sources of support
Canadian Institutes of Health Research and the Nutricia Research Foundation for research funding, and Europharm International Canada Inc. for the in-kind support of supplements.
Additional information
Trial registration clinical trials.gov Identifier NCT00381914
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 83 kb)
Rights and permissions
About this article
Cite this article
Gallo, S., Hazell, T., Vanstone, C.A. et al. Vitamin D supplementation in breastfed infants from Montréal, Canada: 25-hydroxyvitamin D and bone health effects from a follow-up study at 3 years of age. Osteoporos Int 27, 2459–2466 (2016). https://doi.org/10.1007/s00198-016-3549-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3549-z