Abstract
Summary
This study evaluates the incidence of bone fractures in women with BC.We found that women with invasive breast cancer are at an increased risk for bone fractures, with fractures most commonly occurring at lower extremity and vertebral sites. The risk is further increased in women undergoing cancer therapy.
Introduction
Bone loss and fractures in breast cancer have generally been attributed to aromatase inhibitor use. This study assessed the incidence of fractures after invasive breast cancer diagnosis and evaluated bone density and FRAX risk calculation at time of fracture occurrence.
Methods
Retrospective cohort study of women with invasive breast cancer [June 2003–December 2011] who participated in an academic hospital based genetic biobank. Demographic and clinical characteristics were abstracted from the electronic medical record (EMR).
Results
A total of 422 women with invasive breast cancer were assessed; 79 (28 %) sustained fractures during the observation period; fractures occurred at multiple skeletal sites in 27 cases (116 fractures). The incidence of fractures was 40 per 1000 person-years. Women who sustained fractures were mostly white and had a family history of osteoporosis (36.9 %, p = 0.03) or history of a prior fracture (6/79, p = 0.004). Fractures occurred 4.0 years (range 0–12 years) after cancer diagnosis. Fracture cases had femoral neck bone mineral density (BMD) of 0.72 + 0.12 g/cm2, T-score of −1.2, that is, within the low bone mass range. Fractures most commonly occurred in lower extremities, vertebral, and wrist sites. Hip fractures accounted for 11 % of fractures, occurring at a median age of 61 years.
Conclusions
Fractures occur shortly after commencing cancer therapy. Rapid bone loss associated with cancer therapy may precipitate fractures. Fractures occur at relatively higher BMD in BC. Occurrence of fractures in invasive breast cancer raises the possibility of cancer-induced impairment in bone quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The bone effects of early menopause, chemotherapy, and aromatase inhibitors, like those of other serious adverse drug reactions (sADRs), are important and frequently unrecognized causes of morbidity and mortality among cancer patients. Furthermore, a median of 7 years has been reported to elapse before sADRs are communicated to prescribers by pharmaceutical suppliers or the Food and Drug Administration (FDA) [1]. Detection of sADR safety signals for oncology therapies is especially problematic because of the complexity of obtaining high-quality reports of the clinical events associated with sADRs and difficulty in distinguishing between the underlying cancer, comorbid illness, and a toxic effect for a given cancer therapy.
One fourth of the cases of breast cancer in the USA occur before menopause [2]. Chemotherapy-induced premature menopause [3] results in rapid changes in BMD of lumbar spine and femoral neck. Women with chemotherapy induced menopause exhibit a 5.9 % (lumbar spine) and 2.0 % (femoral neck) BMD loss at 12 months and loss of 9.5 % in the lumbar spine and 4.6 % in the femoral neck at 2 years. In contrast, patients with transient chemotherapy-associated amenorrhea and preserved menstruation have only marginal changes in BMD [4]. Especially at risk of bone loss are women in their 40s, as they are more susceptible to developing menopause [4].
Tamoxifen preserves BMD at the spine and hip in postmenopausal women with BC; the beneficial effect lasted for more than 5 years. The mechanism of the protective effect is considered to be the estrogenic action of tamoxifen on tissues other than breast. In premenopausal women, tamoxifen decreases BMD. Tamoxifen has been linked to increased risk for spine and femoral fractures [5, 6].
Adjuvant hormone therapy contributes to cancer treatment-induced bone loss (CTIBL) [7]. Aromatase inhibitors (AI) block estrogen production in peripheral tissues, and the third generation AIs reduce circulating estrogen levels [8, 9]. In recent years, the clinical superiority and economic benefit of AIs over tamoxifen has resulted in increased use of these agents. Clinical trials have shown, however, that AI use results in accelerated bone loss, osteoporosis, and fractures [9–12]. While hip fractures have occurred in less than 1 % of women in randomized clinical trials, between 1996 and 2011 [10], more than 50 reports of hip and femur fractures among middle-aged community-dwelling women (40–64 years of age) were filed with the FDA’s Adverse Event Reporting System (FAERS) [13].
Clinical trials have demonstrated the efficacy of bisphosphonates [14–19] and receptor activator of nuclear factor kappa B ligand (RANKL)-binding agents [20, 21] in preventing bone loss during adjuvant therapy. Denosumab showed efficacy preventing vertebral fractures in cancer patients [21]. The identification of individuals with cancer who are at high risk of fractures is important as pharmacologic treatment can reduce fracture risk. Despite CTIBL rates of bone loss being tenfold higher than normal, few women have clinical risk factor assessment through BMD testing prior to initiation of cancer therapy. Thus, clinicians are not able to identify women who are likely to develop accelerated bone loss during initial cancer therapy.
The National Comprehensive Cancer Network (NCCN) guidelines recommend that postmenopausal women with ER-positive breast cancer consider BMD testing prior to AI therapy. It is further recommended that women in the pre- or peri-menopausal phase be treated with tamoxifen for 5 years. Clinicians are advised to consider sADRs, patient preferences, and pre-existing conditions when determining appropriate adjuvant endocrine therapies. Osteoporosis therapy is recommended in women with osteoporosis or with osteopenia and a 10-year risk (FRAX) of all major fractures of 20 % or hip fractures of 3 % [22].
The objective of our study was to evaluate the incidence of fractures and correlates of fracture risk in a cohort of women with breast cancer included in the NUgene biobank and followed at the Robert H. Lurie Comprehensive Cancer Center (RHLCCC).
Methods—participants
This retrospective cohort study was approved by the Northwestern University Institutional Review Board. The RHLCCC of Northwestern University is one of 41 NCI-designated comprehensive cancer centers. RHLCCC and its affiliated hospitals and practices annually treat more than 10,000 new cancer cases, providing an extensive range of treatment and clinical trial options.
Datasets
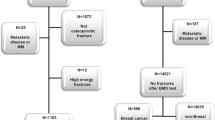
The NUgene Project of the Center for Genetic Medicine collects and stores DNA samples and associated health information from participants at Northwestern-affiliated hospitals and clinics. Participant de-identified health information from the electronic medical record (EMR) and a questionnaire with demographic, health information, and personal and family history of disease are available to investigators. We analyzed the incidence of fractures in women with invasive breast cancer (BC) cases enrolled in NUgene. Enrollment: The study population included women with invasive BC aged 40–89 years of age. Women with metastatic BC on intravenous bisphosphonates were excluded. Participants were selected if they had at least two visits beyond the diagnosis of invasive BC. Data abstraction process: Medical information was obtained from The Northwestern Medicine™ Enterprise Data Warehouse (NMEDW), a cross-institutional effort aimed comprising Northwestern University Feinberg School of Medicine, Northwestern Medical Faculty Foundation, and Northwestern Memorial Healthcare Corporation and is designed to integrate aimed at integrating clinical and administrative data across the Northwestern Medical enterprise to facilitate research, clinical quality, healthcare operations, and medical education. NUgene conducted queries within the NMEDW of discrete data from the aforementioned sources. Individual medical record reviews were conducted in selected cases. The database search spanned data from June 2003 through December 2011. A standardized case report form was used to collect data, comprising clinical risk factors such as smoking, prior fractures, maternal hip fractures, corticosteroid use, and rheumatoid arthritis, and FRAX scores were calculated.
Fracture ascertainment
Fracture occurrence was obtained from the ICD codes in the electronic medical record, and diagnostic codes were derived from radiologic reports noting the presence of a new fracture. Cases with radiographic evidence of pathologic fractures in radiographs, MRI, CT, and PET scans were excluded. BMD results were evaluated immediately preceding the fracture. For women without a fracture, BMD tests done after invasive BC diagnosis were analyzed. Chemotherapeutic agents and adjuvant therapy were abstracted from the medical records and the tumor registry. The study cohort was followed for a minimum of 6 years.
Bone densitometry
Bone densitometry of the lumbar spine and non-fractured hips was performed at the Bone Health Osteoporosis Center using dual-emission X-ray absorptiometry (DXA) technology (Hologic W, Discovery Corp, Waltham, MA). Patients were considered to have osteoporosis if their adjusted T-scores were equal to or less than −2.5 at any measurement site. Normative databases from NHANES III were used to determine T-scores. Data from lumbar spine scans were used only if at least two vertebrae were visualized without interfering artifacts. The coefficient of variation (CV%) was 1 % at spine and 1.5 % at the hip.
Analysis
Sample sizes had 80 % power to detect a mean difference between groups of 0.35 standard deviations, assuming a two-tailed test and a type I error rate of 5 %. Means and standard deviations are reported for continuous variables, and differences are tested via the t test. Frequencies and percentages are reported in Table 2 for categorical variables, and differences were tested via Fisher’s exact test. Cox proportional hazards was conducted to assess risk factors association with fractures. All tests are two-tailed and p value of ≤0.05 was considered statistically significant. No adjustments were made for multiple significance testing.
Results
A total of 422 women with invasive BC were assessed; 79 women (18 %) sustained 116 fractures with multiple fractures occurring in 27 cases. Participants were followed for a total of 1872.55 person-years; the incidence of fractures was 40.2 per 1000 person-years. The control group was comprised of the 343 women with BC who did not fracture. The study population ethnicity was predominantly white (n = 226, 77 %). Clinical risk factors were similar in both groups (Table 1). The majority of women (91 %, n = 72) who sustained a fracture had a BMD T-score > −2.5, and 51 % (n = 40) had a BMD T-score > −1.0. Ten percent of women with fractures had osteoporosis as did 2.5 % of the control group (p = 0.63), 65 % of women with fractures had low bone mass, as did 26 % of the control group (p = 0.83). Women 65 years of age and older had a prevalence of fractures similar to that of the younger cohort. The majority of women were in earlier stages of cancer (stage 0–3), American Joint Committee on Cancer (AJCC) grade 0–3; 33 BC cases were hormone-positive cases (Table 2). Nine women sustained hip fractures at a median age of 61 years (age range of 34–90 years), median femoral neck BMD of 0.746 g/cm2, and a T-score of −1.1 (range T-score from +1.0 to −3.0). The most common sites of fracture were ankle and tibia (26 %), vertebral (13 %), hip (11 %), and wrist 10 % among others.
Chemotherapy
Most commonly used agents were doxorubicin, cyclophosphamide, paclitaxel, carboplatin, trastuzumab, and taxanes. Hormonal therapy included leuprolide, goserelin, tamoxifen, anastrozole, letrozole, and exemestane. Aromatase inhibitors were used in 23 cases (29 %) of the women with invasive BC who sustained fractures.
The baseline characteristics of fracture participants revealed a younger age at menopause and more advanced stage of breast cancer. Univariate Cox proportional hazards analysis demonstrated that earlier age at initiation of calcium supplementation may be protective (Table 3).
Discussion
Our study confirms an elevated incidence of fractures in women with invasive breast cancer. We identified an incidence of fractures of 40.2 % per 1000 person-years in mid-life and older women occurring as early as 4 years post breast cancer diagnosis, that is, somewhat higher than the fracture incidence seen in the aromatase clinical trials [23]. We identified that fractures occur at higher BMD in women with BC than in women with postmenopausal osteoporosis.
The majority of fractures occurred in women with normal or low bone mass; 91 % had a T-score > −2.5 and 55 % of them a normal BMD. Many of these women would therefore not be eligible for bone loss prevention per current clinical guidelines. In the literature, some patients with T-scores above −2.5 have experienced non-traumatic fractures. In the Rotterdam study of 7806 individuals aged 55 years and older, non-vertebral fractures occurred in 56 % of women, with a T-score above ≥ −2.5 [24]. In the Study of Osteoporotic Fractures, 54 % of patients with non-vertebral fracture had a T-score ≥ −2.0 [25]. Bisphosphonate use in CTIBL clinical trials selected women with T-score below −1.0. Two possibilities should be considered, the first is that women with BC may have higher BMD at the time of diagnosis or that an alteration in bone quality may be playing a role in fracture occurrence.
Women with BC may exhibit higher BMD at time of diagnosis. Several studies have demonstrated an association between higher BMD and the risk of BC. In the Study of Osteoporotic Fractures, women with BMD above the 25th percentile were at 2.0 to 2.5 times increased risk of BC as compared with women below the 25th percentile [26]. In a nested case control study of the Marburg breast cancer and osteoporosis trial, it was evidenced that women with BC were more likely to have BMD in the highest quartile [27]. In the Framingham study, the risk of BC increased from 1.0 to 3.5 from the lowest to the highest quartile of BMD [28]. Therefore, our findings that women with BC may sustain fractures at higher BMD could be explained by a relatively higher baseline BMD, and though chemotherapy may cause BMD decline or change in bone quality, women would fracture at relatively higher BMDs as compared to postmenopausal women.
Another aspect to consider is that bone strength is determined by BMD and bone quality. Bone quality depends on bone microarchitecture and rate of bone turnover (remodeling). Alterations in bone microarchitecture have been identified in individuals with fractures, independently of BMD [29]. Some conditions that exhibit fracture occurrence at higher BMDs are diabetes mellitus and glucocorticoid treatment [29–31]. Bone microarchitecture has better identified subjects that fracture as compared to those who remain fracture free, independent of BMD. Some examples include the Multiple Outcome in Raloxifene Evaluation (MORE) clinical trial where bone biopsies showed greater disruption of the trabecular lattice in terms of total strut length per area and trabecular bone pattern factor in women with vertebral fractures [32]. Chronic glucocorticoid treatment has also been associated with a rapid and significant bone loss characterized by a major loss of trabecular connectivity, which may in part explain the increased vertebral fracture risk that occurs at higher BMD thresholds in glucocorticoid-induced osteoporosis than in postmenopausal osteoporosis [33]. We hypothesize that fractures in women with breast cancer reflect such causes as an alteration in bone quality. This would include alterations in bone microarchitecture and composition and increased bone resorption due to cancer or therapy. Chemotherapy could be inducing osteoblast apoptosis and upregulation of osteoclast resorption. In terms of bone microarchitecture, changes could have included effects on trabecular bone such as thinning of trabeculae, increased microfractures, loss of connectivity, and effects on cortical bone such as a decrease in cortical thickness and an increase in cortical porosity. These multiple changes would contribute to impaired bone biomechanical strength [34].
Few studies have explored the effect of cancer therapy on bone microarchitecture. In a bone strength substudy of a primary prevention of BC in at risk women (MAP 3 trial), exemestane, reduced total volumetric BMD and decreased cortical thickness and increased cortical porosity at the tibia and radius and decreased areal BMD at the spine and hip over 2 years [35]. A substudy of the randomized Tamoxifene Exemestane Adjuvant Multinational (TEAM) trial to determine the effects of these agents on bone quantity and quality was conducted using trabecular bone score (TBS). Exexemstane resulted in decreases in bone quantity and quality, where tamoxifen induced an increase in bone quantity and quality [36]. A bone microarchitecture study also confirmed that high-dose bisphosphonate use in women with BC for 20 months failed to alter bone microarchitecture. Women on long-term bisphosphonate therapy for breast cancer had normal BMD by DXA and normal cortical and trabecular volumetric BMD, cortical thickness and trabecular number at the peripheral skeleton compared to healthy young women and age-matched women [37].
The most common sites of fracture included lower extremity and vertebral sites. Although lower extremity fractures are not considered the typical “osteoporotic” fractures, ankle fractures are associated with alterations in bone microarchitecture resulting in greater bone stiffness and result in a higher risk for fractures [38, 39]. Lower extremity fractures may predispose to falls and hip fractures in older adults [40]. Vertebral bone loss and fractures are likely due to rapid trabecular resorption after menopause and chemotherapy. Additionally, an element of radiation effect may occur as most women undergo radiation therapy [41], despite shielding techniques. Trabecular bone as seen in vertebral bodies is metabolically active and responds rapidly to metabolic changes. More than 10 % of women with BC had sustained a hip fracture. Surprisingly, in women with breast cancer, the median age at which hip fracture occurred was 56 years of age. This compares to the mean age of occurrence in women without breast cancer at 74 years of age [42]. Thus, hip fractures are occurring 20 years earlier than anticipated.
Wrist fractures are most commonly seen in the early postmenopausal years when women are still fit enough to respond to a fall with protective responses (using arms to slow down fall, or protect their face). It is evident that fractures occurring in BC patients are typically “osteoporotic” in distribution and not due to high-impact trauma. This may appear to conflict with a relatively “normal’ BMD, supporting the notion of an impairment in bone quality. Typically, vertebral fractures occur during normal activities, being asymptomatic in most cases and merely manifesting as height loss. In comparison, hip fractures occur when balance mechanisms are overcome and the individual falls without being able to mount protective responses [43].
We were not able to identify a specific chemotherapeutic regimen that was uniquely associated with fracture occurrence. Remarkably, aromatase inhibitors were used in a low proportion of patients and may explain the lack of association with fractures. It would appear that such fractures are multifactorial in origin, including premature menopause, chemotherapy effect, and possibly the use of adjuvant therapy. Bisphosphonates were more frequently used by women who had sustained a fracture. Several agents have been studied for prevention and treatment of cancer and treatment-induced bone loss including bisphosphonates, such as zoledronic acid, and risedronate [44], as well as a receptor activator of nuclear factor kappa B ligand (RANKL) inhibitor, the monoclonal antibody, denosumab. Denosumab has been shown to prevent vertebral fractures and has recently gained FDA approval for treatment of patients at high risk for fractures and use of androgen deprivation in non-metastatic prostate cancer or adjuvant aromatase inhibitor therapy for breast cancer [20].
The World Health Organization fracture risk assessment tool (FRAX) is based on clinical risk factors identified from multiple epidemiologic studies of individuals without cancer and provides clinicians with a 10-year estimate for major fractures (wrist, hip, and spine) and for a 10-year risk of hip fracture. The current threshold for treatment in the USA is a 10-year risk of major fractures ≥20 % or a risk for hip fractures ≥3 %. In women with breast cancer, FRAX scores were similar in women with and without fractures. Thus, we can conclude that in our study, BMD and FRAX risk calculation were of modest benefit.
Limitations of our study include the retrospective nature of this analysis; we recognize that the incidence of fractures may be overestimated, as women with greater genetic concerns would be more likely to participate in NUgene. However, fracture events seen in other institutions would not be included in the EDW which would lead to an underestimation. Our work focused on mid-life and older community-dwelling women; thus, our findings cannot be extrapolated to all older women with breast cancer and other comorbidities. This study may not be generalized to all clinical settings as ours was a single academic site. Bisphosphonates were frequently used in this cohort. Although drug adherence was not confirmed, we consider the association of bisphosphonates with fracture occurrence to be spurious in nature.
In conclusion, the incidence of fractures in women with breast cancer in the study is high and fractures may occur as early as 4 years after cancer diagnosis, prior to the use of aromatase inhibitors. Conventional risk factors for fractures, BMD, and the fracture risk calculation assessment, FRAX, were not highly predictive for fractures. Early age at onset of calcium supplementation was protective. Alternative mechanisms for imaging and evaluation of bone strength are required in women with breast cancer. Additional research in the area is needed.
References
Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH (2002) Timing of new black box warnings and withdrawals for prescription medications. Jama 287(17):2215–2220
Jemal A, Tiwari RC, Murray T et al (2004) Cancer statistics. CA Cancer J Clin 54:8–29
Shapiro CL, Manola J, Leboff M (2001) Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 19(14):3306–3311
Saarto T, Blomqvist C, Valimaki M, Makela P, Sarna S, Elomaa I (1997) Chemical castration induced by adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy causes rapid bone loss that is reduced by clodronate: a randomized study in premenopausal breast cancer patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 15(4):1341–1347
Ding H, Field TS (2007) Bone health in postmenopausal women with early breast cancer: how protective is tamoxifen? Cancer Treat Rev 33(6):506–513
Cooke AL, Metge C, Lix L, Prior HJ, Leslie WD (2008) Tamoxifen use and osteoporotic fracture risk: a population-based analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 26(32):5227–5232
Guise TA (2006) Bone loss and fracture risk associated with cancer therapy. Oncologist 11(10):1121–1131
Miller WR (1996) Aromatase inhibitors—where are we now? Br J Cancer 73(4):415–417
McCloskey EV (2006) Effects of third-generation aromatase inhibitors on bone. Eur J Cancer 42:1044–1051
Baum M, Buzdar A, Cuzick J et al (2003) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial efficacy and safety update analyses. Cancer 98(9):1802–1810
Thurlimann B, Hess D, Koberle D et al (2004) Anastrozole ('Arimidex') versus tamoxifen as first-line therapy in postmenopausal women with advanced breast cancer: results of the double-blind cross-over SAKK trial 21/95—a sub-study of the TARGET (Tamoxifen or ‘Arimidex’ Randomized Group Efficacy and Tolerability) trial. Breast Cancer Res Treat 85(3):247–254
Thurlimann B, Keshaviah A, Coates AS et al (2005) Breast International Group (BIG) 1–98 Collaborative Group. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353:2747–2757
Edwards BJ, Raisch DW, Shankaran V et al (2011) Cancer therapy associated bone loss: implications for hip fractures in mid-life women with breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 17(3):560–568
Delmas PD, Balena R, Confravreux E, Hardouin C, Hardy P, Bremond A (1997) Bisphosphonate risedronate prevents bone loss in women with artificial menopause due to chemotherapy of breast cancer: a double-blind, placebo-controlled study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 15(3):955–962
Brufsky A (2011) Adjuvant bisphosphonates for early-stage breast cancer. Lancet Oncol 12(7):610–611
Brufsky A, Harker WG, Beck JT et al (2007) Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 25(7):829–836
Coleman R (2007) Potential use of bisphosphonates in the prevention of metastases in early-stage breast cancer. Clin Breast Cancer 7(Suppl 1):S29–S35
Hershman DL, McMahon DJ, Crew KD et al (2008) Zoledronic acid prevents bone loss in premenopausal women undergoing adjuvant chemotherapy for early-stage breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 26(29):4739–4745
Lester JE, Dodwell D, Purohit OP et al (2008) Prevention of anastrozole-induced bone loss with monthly oral ibandronate during adjuvant aromatase inhibitor therapy for breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 14(19):6336–6342
Ellis GK, Bone HG, Chlebowski R et al (2008) Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 26(30):4875–4882
Lipton A, Steger GG, Figueroa J et al (2007) Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 25(28):4431–4437
Gralow JR, Biermann JS, Farooki A et al (2009) NCCN Task Force Report: bone health in cancer care. J Natl Compr Canc Netw 7(3):S1–S32, quiz S33-35
Howell A, Cuzick J, Baum M et al (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365(9453):60–62
Schuit SC, van der Klift M, Weel AE et al (2004) Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone 34(1):195–202
Wainwright SA, Marshall LM, Ensrud K et al (2005) Hip fracture in women without osteoporosis. J Clin Endocr Metab 90:2787–2793
Cauley JA, Lucas FL, Kuller LH, Vogt MT, Browner WS, Cummings SR (1996) Bone mineral density and risk of breast cancer in older women: the study of osteoporotic fractures. Study of Osteoporotic Fractures Research Group. Jama 276(17):1404–1408
Hadji P, Gottschalk M, Ziller V, Kalder M, Jackisch C, Wagner U (2007) Bone mass and the risk of breast cancer: the influence of cumulative exposure to oestrogen and reproductive correlates. Results of the Marburg breast cancer and osteoporosis trial (MABOT). Maturitas 56(3):312–321
Zhang Y, Kiel DP, Kreger BE et al (1997) Bone mass and the risk of breast cancer among postmenopausal women. N Engl J Med 336(9):611–617
Boutroy S, Bouxsein ML, Munoz F, Delmas PD (2005) In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90(12):6508–6515
Burghardt AJ, Issever AS, Schwartz AV et al (2010) High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 95(11):5045–5055
Vestergaard P, Rejnmark L, Mosekilde L (2009) Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcif Tissue Int 84(1):45–55
Oleksik A, Ott SM, Vedi S, Bravenboer N, Compston J, Lips P (2000) Bone structure in patients with low bone mineral density with or without vertebral fractures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 15(7):1368–1375
Luengo M, Picado C, Del Rio L, Guanabens N, Montserrat JM, Setoain J (1991) Vertebral fractures in steroid dependent asthma and involutional osteoporosis: a comparative study. Thorax 46(11):803–806
Guo XE, Kim CH (2002) Mechanical consequence of trabecular bone loss and its treatment: a three-dimensional model simulation. Bone 30(2):404–411
Cheung AM, Robbins J, Pruthi S, et al. Cortical porosity and estimated bone strength in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: Analyses from the nested bone strength substudy of the map.3 trial (MAP3BSS). Journal of Bone and Mineral Research. 2012;27.
Hadji P, Kalder M, Kauka A, Bauer M, Ziller M, Hans D (2012) Effects of exemestane and tamoxifen treatments on bone quantity and quality in patient with breast cancer. Osteoporos Int 23:S289–S290
Shao T, Shane ES, McMahon D et al (2012) Effects of high dose of bisphosphonate therapy on bone microarchitecture of the peripheral skeleton in women with early stage breast cancer. Cancer Res 72:6–12, 03
Stein EM, Liu XS, Nickolas TL et al (2011) Abnormal microarchitecture and stiffness in postmenopausal women with ankle fractures. J Clin Endocrinol Metab 96(7):2041–2048
Warriner AH, Patkar NM, Curtis JR et al (2011) Which fractures are most attributable to osteoporosis? J Clin Epidemiol 64(1):46–53
Liu-Ambrose T, Eng JJ, Khan KM, Carter ND, McKay HA (2003) Older women with osteoporosis have increased postural sway and weaker quadriceps strength than counterparts with normal bone mass: overlooked determinants of fracture risk? J Gerontol A Biol Sci Med Sci 58(9):M862–M866
Wernle JD, Damron TA, Allen MJ, Mann KA (2010) Local irradiation alters bone morphology and increases bone fragility in a mouse model. J Biomech 43(14):2738–2746
Johnell O, Kanis J (2005) Epidemiology of osteoporotic fractures. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 16(Suppl 2):S3–S7
Cummings SR, Nevitt MC (1994) Non-skeletal determinants of fractures: the potential importance of the mechanics of falls. Study of Osteoporotic Fractures Research Group. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 4(1):67–70
Body JJ, Bergmann P, Boonen S, et al. Management of cancer treatment-induced bone loss in early breast and prostate cancer—a consensus paper of the Belgian Bone Club Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2007;epub.
Acknowledgments
The authors acknowledge Tina Kiguradze for her editorial assistance. All authors of this research paper have directly participated in the planning, execution, or analysis of the study. All authors of this paper have read and approved the final version submitted.
The table below summarizes each author’s role in preparing this manuscript
Authors | Concept/study design | Data collection/interpretation | Drafting manuscript | Critical revision of manuscript | Statistical analysis | Funding | Administrative, technical, or material support |
Beatrice J Edwards | Yes | Yes | Yes | Yes | Yes | ||
William Gradishar | Yes | Yes | Yes | ||||
Jennifer Pacheco | Yes | Yes | Yes | ||||
Maureen Smith | Yes | Yes | Yes | Yes | |||
Jaime Holbrook | Yes | Yes | Yes | ||||
June McKoy | Yes | Yes | Yes | Yes | |||
Beatrice Nardone | Yes | Yes | Yes | ||||
Stefani Tica | Yes | Yes | Yes | ||||
Victoria Godinez Puig | Yes | Yes | Yes | ||||
Alfred Rademaker | Yes | Yes | Yes | ||||
Irene Helenowski | Yes | Yes | Yes | ||||
Andrew D. Bunta | Yes | Yes | Yes | Yes | |||
Paula Stern | Yes | Yes | Yes | Yes | |||
Steven Rosen | Yes | Yes | Yes | Yes | |||
Dennis West | Yes | Yes | Yes | Yes | Yes | ||
Theresa Guise | Yes | Yes | Yes |
This work was supported by the National Institute of Health [grants 5R01-CA125077-03 (DPW), 3R01CA125077-03S1 (BJE), and 5K01 CA134554-05 (JMM)]. A portion of this work was presented at the Annual Conference on Cancer and Bone in Chicago, December 9–11, 2011, sponsored by the International Bone and Mineral Society (IBMS), and the ASCO Breast Symposium September 6–7, 2013 San Francisco, California.
Funding sources
This work was supported by: National Institute of Health [grants 5R01-A125077-03 (DPW), 3R01CA125077-03S1 (BJE) and 5 K01 CA134554-05 (JMM)].
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Edwards, B.J., Gradishar, W.J., Smith, M.E. et al. Elevated incidence of fractures in women with invasive breast cancer. Osteoporos Int 27, 499–507 (2016). https://doi.org/10.1007/s00198-015-3246-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3246-3