Abstract
Summary
This study describes bone mass changes during pregnancy and lactation measured by a special ultrasound method. Pregnant women showed a decrease of bone mass followed by a stable bone mass while breast-feeding afterwards. Later in life, there is a recovery of bone mass loss.
Introduction
The aim of this study was to evaluate bone changes during pregnancy using the radiation-free method of quantitative ultrasonometry (QUS).
Methods
One hundred twenty-five pregnant women who underwent prenatal care were included in this study. Ultrasound measurement of the calcaneus was performed in each trimester and then 6 weeks, 3 months, and 1 year postpartum. The calcaneal QUS measurements were carried out using the Achilles plus device (GE/Lunar Corporation, Madison, WI). Three ultrasound variables were measured: speed of sound (SOS, m/s), broadband ultrasound attenuation (BUA, dB/MHz), and the “stiffness index” (expressed as the percentage of the mean value in young adults). SOS and BUA raw data result in the t-score and z-score.
Results
A complete panel of six measurements was acquired over the time period in 101 patients (80.8 %). Forty-two percent of the included patients were primipara, while 58 % had given birth to at least one child (47 %) previously. There was a statistically significant change of the t-score (tv = 2.14, p = 0.035) and the stiffness index (tv = 2.46, p = 0.016) from the second to the third trimester, followed by a plateau during lactation. Interestingly, the t-score remained stable during lactation, regardless of the duration of lactation (<3 months, 3–6 months, and >6 months).
Conclusions
Young primiparas who had a sedentary adolescence were at the highest risk of bone loss during pregnancy. Bone loss that occurred during pregnancy was typically recovered later on, based on unknown molecular and biochemical mechanisms that must be elucidated with further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is one of the ten most common diseases. Increased bone remodeling that results in the loss of bone mineral density and destruction of the bone microarchitecture can be found in postmenopausal women due to estrogen deficiency (prevalence: 7 % of all 55-year-old postmenopausal women) [1]. Osteoporosis typically causes fractures of the spine or hip with a massive effect on the quality of life. This leads to permanent or long-term care as well as increasing costs for the health system [2]. The official WHO definition is based on the central dual-energy X-ray absorptiometry (DXA). The t-score represents an equation of these radiation measurements within a collective of young 30-year-old women. Fulfilling the criteria of osteoporosis [1] requires the score to be below −2.5 standard deviations. In addition, the Fracture Risk Assessment Tool (FRAX) is an algorithm including clinical risk factors and a hip DXA scan to predict a patient’s 10-year probability of having an osteoporotic fracture [3].
Generally, the diagnosis and treatment of osteoporosis refers to postmenopausal women. Interestingly, there is a small subgroup of young premenopausal pregnant women also affected by osteoporosis. However, these patients suffering from pregnancy-associated osteoporosis are commonly undiagnosed and not treated accordingly [4, 5]. They complain about severe pain from vertebral fractures and are unable to take care of the newborn [6]. The pathological mechanism of this disease is completely unknown, precisely because most of the patients do not present the classical well-known risk factors of postmenopausal osteoporosis [7]. One possible correlation can be seen in the increased demand of calcium in order to form the fetal skeleton in pregnancy. The full-term neonate contains about 30 g of calcium, and theoretically, to fulfill this requirement of calcium, the bone mass of the mother can decrease, especially in the last trimester of pregnancy. Hypercalciuria can be observed during pregnancy as a sight of enhanced calcium mobilization [8]. Compensatory mechanisms exist to counter this, such as elevated estrogen levels during the course of pregnancy, an intestinal increase of calcium absorption, and possibly renal conservation. The aim of this study is to evaluate signs of maternal bone loss during pregnancy using the radiation-free method of quantitative ultrasonometry (QUS) [9]. In literature, the direct comparison of DXA with QUS of the calcaneus shows a positive correlation between both methods [10]. Therefore, QUS may be able to prove changes during the physiological course of pregnancy.
Materials and methods
Subjects
One hundred twenty-five pregnant Caucasian women who underwent standard prenatal care at a gynecological practice near Frankfurt am Main, Germany, were included in this study. The routine checkup of prenatal care has been extended to an ultrasound measurement of the calcaneus in each trimester, 6 weeks, 3 months, and 1 year postpartum. All participants gave informed consent and completed a detailed questionnaire on the patient’s obstetric history, osteoporosis risk factors, as well as lifestyle factors that are known to influence the bone metabolism. The patient’s age and all physiognomic data such as height, weight, and the resulting body mass index (BMI) were documented, and the age of menarche, the application of oral contraceptives, as well as the duration of a possibly secondary amenorrhea and irregularities in the menstruational cycle were questioned. The patient’s family and own history on malignancies including their therapy, occurrence of thrombosis, thyroid dysfunction, all types of allergies, lactose intolerance, as well as osteoporosis of close relatives, previous bone fractures, and treatment with cortisone, nicotine and/or alcohol abuse, and exercise was assessed. Moreover, the number of pregnancies, miscarriages, and abortions, as well as fertility treatment in the current pregnancy and the period of breastfeeding was documented in this study.
Ultrasound device
The calcaneal QUS measurements were carried out using the Achilles plus device (GE/Lunar Corporation, Madison, WI). This system includes two unfocused transducers (2.5-cm diameter) mounted approximately 9.5 cm apart. One of the transducers represents the transmitter while the other one works as the receiver. After the patient’s heel is immersed in a 35 °C-heated 100-cm3 water bath, acoustic coupling is transmitted. Two ultrasound variables were measured: speed of sound (SOS, m/s) and broadband ultrasound attenuation (BUA, dB/MHz) [11]. This data results in the t-score and z-score, which appear in the following tables. The third measured variable is the so called “stiffness index” (SI, expressed as the percentage of the mean value in young adults). It has a higher degree of predictability of fractures than SOS and BUA. The SI is calculated by applying the following formula: SI = 0.67 × (BUA) + 0.28 × (SOS) − 420. A daily quality control using a standardized model was performed before any measurement in vivo. The short-term precision in vivo in 31 adults was 0.2, 1.2, and 1.3 for SOS, BUA, and SI, respectively [12].
Statistical analysis
Statistical analysis was performed by SPSS (IBM) for Windows version 20. Statistical significance was achieved with a p value <0.05. Normal distribution was evaluated with the Kolmogorov-Smirnov test. The paired t test and the Wilcoxon test were used to test mean differences for flow statistics and the U test for non-parametric comparison.
Results
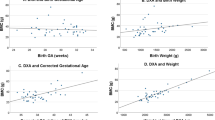
One hundred twenty-five pregnant women who consulted the gynecologist for the first checkup in this pregnancy were recruited for the study. The investigation features high compliance because all six measurement values were completed over the study period in 101 patients (80.8 %). Patients were excluded from the study in case of a miscarriage, pre-term delivery, or relocation from the previous residence. The mean age of the study’s participants was 32.2 years (4.8 ± SD) and the body mass index (BMI) 23.7 (4.4 ± SD). The distribution of age and BMI is represented in Figs. 1 and 2. The factor age correlates to a normal Gaussian distribution. With respect to the correct classification of the data in relation to the conception or to the real date of birth, a deviation of 20 % of the defined point of measurement was tolerated. The mean follow-up dates in the course of pregnancy were 10.6 weeks of pregnancy in the first trimester, 20.9 weeks in the second trimester, and 30.6 weeks in the third trimester. The postpartum checkup appointments were 6.2 weeks, 6.2 months, and 12.2 months after delivery, on average.
Forty-two percent of the included patients were primipara, while 58 % had given birth to at least one child. Forty-seven percent of the patients have born one child, 9 % two children, and 2 % three children. Thirty-six percent of the multiparous women had exclusively breastfed their children for not less than 6 months. Due to the present evaluated pregnancy, 51 % of the women had nursed their newborns exclusively for 6 months. Fertility treatment was successfully performed in 6 % of the patients.
The family and the patient’s history of the 101 study participants present a non-homogeneous picture that can be divided into numerous subgroups. Unfortunately, the sample size in these subgroups is too small to perform a statistical evaluation. None of the patients took a medication that is known to influence bone metabolism. Nine percent of the participants had a family history of osteoporosis. Regular physical activity with a mean of 3 hours per week was performed by 46 % of the patients. Fifty-nine percent of participants are predominantly seated and therefore inactive while working, while only 11 % claim to be physically active during their job. Nutritional habits and social drugs can be neglected, as 6 % of the women stated to have lactose intolerance and 11 % have declared to be ex-smokers.
All recorded values at the six measurement points of time (first, second, and third trimester and 6 weeks, 6 months, and 12 months postpartum) showed a normal distribution using the Kolmogorov-Smirnov test. Statistical evaluation was performed using parametric tests, whereby each QUS measurement was compared to the value measured at the prior appointment. It is worth noting that there is a statistically significant change of the t-score (tv = 2.14, p = 0.035) and of the stiffness index (tv = 2.46, p = 0.016) from the second to the third trimester. The data is presented in Table 1. Figure 3 illustrates a significant decrease of the t-score in the third trimester compared to the second, followed by a plateau during the period of breastfeeding. Comparing primipara (n = 42) to multipara (n = 59) only women who gave birth to their first child represented a significant decrease (using the Wilcoxon test) of the t-score (p = 0.003), z-score (p = 0.031), and SI (p = 0.029) between the first trimester and 12 months postpartum. There are no differences in height, weight, BMI, and time of lactation in the subgroups.
Interestingly, there is a significant decrease in SI, t-score, and z-score (p = 0.003, p = 0.006, p = 0.008) in patients with a BMI lower than 25; in obese patients, no significant loss can be noted. In the postpartum period, the BMI is found to have no influence on the outcome.
Primiparas with a history of low exercise during adolescence have the largest risk at losing bone mass density (BMD) during pregnancy and lactation. All measured variables in this subgroup showed a significant decrease in t-score (t value −2.22, p = 0.025), z-score (t value −2.17, p = 0.029), and SI (−2.4, p = 0.016) from the beginning of the pregnancy until 1 year postpartum.
Splitting our cohort of pregnant women by age, younger than 35 years and older than 34 years, it can be noted that patients older than 34 years are exposed to a lower risk of losing bone mass density compared to younger women. The latter show a significant decrease in t-score and SI over the whole period in comparison to the first.
Dividing the lactation period in three time frames (<3 months, 3–6 months, and > 6 months), there can be no difference observed in t-score changes during the postpartum period.
A history of bone fractures was found in 34 patients, but without generalized osteoporosis. Only two patients with one fracture in their history presented a QUS t-score of ≤−2.5 as an initial value. Eighty-two percent of the pregnant women showed normal ultrasound measurement values.
Discussion
Our results indicate a loss of QUS variables during pregnancy, followed by a plateau during the period of breastfeeding. These findings are highlighted by a significant change of the t-score and the stiffness index during pregnancy. Conversely, the period of lactation is not associated with an alteration of these values.
Osteoporosis in this group of pregnant and postpartum women has yet not been investigated thoroughly. There are only a few studies investigating changes in bone mass, bone mass density, or bone metabolism in this subgroup of women [13] . A novelty of this study is the long follow-up over 2 years with a total of six measurements in 101 patients.
Generally, the dual energy X-ray absorptiometry (DXA) is the established method to evaluate bone mineral density changes, but the use of ionizing radiation is an undesirable disadvantage for studies in pregnancy. Although the radiation dose of 0.001 mSv that is used in DXA can be considered as very low, it should be restricted to those pregnant women with suspected pregnancy-associated osteoporosis.
At the beginning of the era of bone mass examination, methods like single-photon absorptiometry (SPA) or DXA studies were performed in pregnant women. Unfortunately, these few studies yield inconsistent results [14–20]. More recent studies indicate a moderate loss of bone mineral density (BMD) of trabecular bone in the spine and hip during pregnancy [21]. If not breastfeeding, a recovery of this loss was uniformly observed. In lactating women, an ongoing decrease of approximately 5 % of BMD of the lumbar spine can be observed. Karlsson’s study confirmed the effect of lactation on lumbar spine mineral density in 73 women, especially during the first 5 months of lactation, with no loss further on (the study was carried on until 12 months). There is no complete recovery of BMD noted within 5 months after weaning off. Interestingly, women with four or more pregnancies had no lower BMD compared to women that gave birth to only one or two babies [22]. A recent study by Mǿller et al. [23] showed a significant decrease of BMD in pregnant women in the lumbar spine (1.8 ± 0.5 %), the hip (3.2 ± 0.5 %), as well as the whole body (2.4 ± 0.3 %) and in the distal forearm (4.2 ± 0.7 %) compared to age-matched controls. BMD was measured seven times before pregnancy and during pregnancy, followed by lactation. A total number of 153 women were included in this study; complete measurements were available in 71 patients. This is the largest longitudinal study in this regard. Postpartum BMD decreased further while breastfeeding. Nine months (n = 31) after giving birth, BMD reverted to baseline values, regardless of the length of breastfeeding [23]. These results correlate to the conclusions of Kalkwarf et al. in 65 women that were breastfeeding. This study exclusively observed the lactation period and excluded the period of pregnancy. In comparison to the age-matched non-lactating controls, a significant loss of total body BMD (−2.8 versus −1.7 %) and lumbar spine BMD (−3.9 versus 1.5 %) could be found. Interestingly, breastfeeding women regained more of their BMD in the lumbar spine compared to non-lactating women after weaning [24]. This suggests a rather protective effect of breastfeeding for osteoporosis [25–29] . In accordance to the findings of Kalkwarf et al., DXA measurement of forearm bone mineral density showed a decrease (statistically non-significant) in the first 6 months of lactation followed by a subsequent recovery of bone mass [30].
Our own preliminary study investigated changes of QUS values in pregnant women without using radiation. In accordance with the study of Aguado et al., we found a significant decrease of speed of sound (SOS) and bone transmission time (BTT) throughout pregnancy. These measurements were performed at each trimester using QUS of the phalanges [31, 32]. This decrease was independent of osteoporosis-related risk factors and the increase in body weight. Although quantitative ultrasonography of the phalanges is able to predict hip, vertebral, and non-vertebral fractures [33], there are more cross-sectional and prospective studies of QUS on the calcaneus that provide evidence of fracture risk prediction somewhat comparable to DXA [34, 35]. Yamaga et al. first published a longitudinal study analyzing speed of sound (SOS) and broadband ultrasound attenuation (BUA) of the os calcis in 18 healthy women throughout pregnancy and puerperium. Even in this small cohort, they found a significant decrease of SOS end BUA in the third trimester compared to the early stage of pregnancy. During a 6-month period of lactation, there was no difference of the ultrasound variables [36]. On the basis of the decrease of ultrasound variables during pregnancy, it could be concluded that there is a loss of bone mass in this period. Additionally, the same study group reports an activation of biochemical markers that correspond with an increase in bone metabolism. Biochemical markers of bone resorption, such as serum type I collagen C-telopeptides (CTX), carboxy-terminal telopeptide of type I collagen (ICTP), and tartrate-resistant phosphatase as well as markers of bone remodeling, such as alkaline phosphatase (AP) and N-terminal pro-peptides of type I collagen (PINP), were found at increased levels [31, 36, 37]. In spite of this high bone turnover during the pregnancy and lactation period, neither pregnancy nor lactation had a significant impact on QUS variables or fracture risk later in life as shown in a multicenter study using matched-pair analysis as well as multiple linear regression analysis in 2080 postmenopausal women [38]. However, there are two current studies based on DXA measurements in postmenopausal women that report different results. Prolonged breastfeeding period per child over 1 year was the highest risk factor for osteoporosis later in life regardless of the patient’s age. Nevertheless, high parity seemed to have a protective effect on bone mass loss [39, 40].
Although our study represents a follow-up of 101 patients with a detailed risk evaluation, this cohort is still too small to generate statistically relevant results in all subgroups. Importantly, we found that women who gave birth to their first child in combination with the lack of exercise in adolescence are at high risk of increased bone loss. These findings correspond to our clinical experience [41, 42]. Younger women are exposed to a greater risk of changes in ultrasound variables. This may be explained by the co-incidence of pregnancy with a not yet established peak bone mass. The fact that obese patients (BMI > 25) are at a lower risk of developing osteoporosis can be explained by the higher estrogen metabolism in the adipose tissue.
Conclusion
Our study demonstrated a significant decrease of the t-score and SI during the course of pregnancy (second to the third trimester). This correlates to the increasing demand of calcium and consecutive loss of BMD during the highest phase of fetal growth at the end of pregnancy. The following plateau during lactation or weaning continued over the whole investigation period of 12 months postpartum. A revert of QUS values was not apparent in our study. As mentioned before, young primiparas lacking exercise in adolescence were at the highest risk of bone loss. The obvious recovery later results from unknown molecular and biochemical mechanisms that require further investigation.
References
Morii H (2005) WHO technical report 921 “prevention and management of osteoporosis”. Clin Calcium 15(4):557–562
Sambrook PN, Cameron ID, Chen JS, Cumming RG, Lord SR, March LM, Schwarz J, Seibel MJ, Simpson JM (2007) Influence of fall related factors and bone strength on fracture risk in the frail elderly. Osteoporos Int 18(5):603–610
Hanson L (2013) Use of the FRAX screening tool to assess fracture risk in a long-term care facility. Consult Pharm 28(7):426–431. doi:10.4140/TCP.n.2013.426
Ferrari S, Bianchi ML, Eisman JA, Foldes AJ, Adami S, Wahl DA, Stepan JJ, de Vernejoul MC, Kaufman JM, IOF Committee of Scientific Advisors Working Group on Osteoporosis Pathophysiology (2012) Osteoporosis in young adults: pathophysiology, diagnosis, and management. Osteoporos Int 23(12):2735–2748. doi:10.1007/s00198-012-2030-x, Epub 2012 Jun 9
Iwamoto J, Sato Y, Uzawa M, Matsumoto H (2012) Five-year follow-up of a woman with pregnancy and lactation-associated osteoporosis and vertebral fractures. Ther Clin Risk Manag 8:195–199. doi:10.2147/TCRM.S30668, Epub 2012 Apr 10
Campos-Obando N, Oei L, Hoefsloot LH, Kiewiet RM, Klaver CC, Simon ME, Zilikens MC (2014) Osteoporotic vertebral fractures during pregnancy: be aware of a potential underlying genetic cause. J Clin Endocrinol Metab 99(4):1107–1111
Smith R, Athanasou NA, Ostlere SJ, Vipond SE (1995) Pregnancy-associated osteoporosis. Q J Med 88:865–878
Sowers M (1996) Pregnancy and lactation as risk factors for subsequent bone loss and osteoporosis. J Bone Miner Res 11(8):1052–1060
Adami S, Giannini S, Giorgino R, Isaia GC, Maggi S, Sinigaglia L, Filipponi P, Crepaldi G (2004) Effect of age, weight and lifestyle factors on calcaneal quantitative ultrasound in premenopausal women: the ESOPO study. Calcif Tissue Int 74(4):317–321
Xu Y, Guo B, Gong J, Xu H, Bai Z (2013) The correlation between calcaneus stiffness index calculated by QUS and total body BMD assessed by DXA in Chinese children and adolescents. J Bone Miner Metab 22
Hadji P, Hars O, Bock K, Albert US, Beckmann MW, Emons G, Schulz KD (1999) Age changes of calcaneal ultrasonometry in healthy German women. Calcif Tissue Int 65:117–120
Kyvernitakis I, Saeger U, Ziller V, Bauer T, Seker-Pektas B, Hadji P (2013) The effect of age, sex hormones, and bone turnover markers on calcaneal quantitative ultrasonometry in healthy German men. J Clin Densit
Holmberg-Marttila D, Sievänen H, Tuimala R (1999) Changes in bone mineral density during pregnancy and postpartum: prospective data on five women. Osteoporos Int 10:41
Christiansen C, Rodbro P, Heinlid B (1976) Unchanged total body calcium in normal human pregnancy. Acta Obstet Gynecol Scand 56:217–219
Lamke B, Brundin J, Moberg P (1977) Changes of bone mineral content during pregnancy and lactation. Acta Obstet Gynecol Scand 56:217–219
Drinkwater BL, Chesnut CH 3rd (1991) Bone density changes during pregnancy and lactation in active women: a longitudinal study. Bone Miner 14:153–160
Sowers MF, Crutchfield MM, Jannausch ML (1991) A prospective evaluation of bone mineral change in pregnancy. Obstet Gynecol 77:841–845
Kent GN, Price RI, Gutteridge DH (1990) Human lactation: forearm trabecular bone loss, increased bone turnover, and renal conservation of calcium and inorganic phosphate with recovery of bone mass following weaning. J Bone Miner Res 5:361–369
Kent GN, Rice RI, Gutteridge DH et al (1993) Effect of pregnancy and lactation on maternal bone mass and calcium metabolism. Osteoporos Int 3(1):44–47
Cross NA, Hillman LS, Allen SH, Krause GF, Viera NE (1995) Calcium homeostasis and bone metabolism during pregnancy, lactation and postweaning: a longitudinal study. Am J Clin Nutr 61:514–523
Oliveri B, Parisi MS, Zeni S, Mautalen C (2004) Mineral and bone mass changes during pregnancy and lactation. Nutrition 20:235–240
Karlsson C, Obrant KJ, Karlsson M (2001) Pregnancy and lactation confer reversible bone loss in humans. Osteoporos Int 12(10):828–834
Mǿller UK, Streym S, Mosekilde L (2012) Changes in bone mineral density and body composition during pregnancy and postpartum. A controlled cohort study. Osteoporos Int 23:1213–1223
Kalkwarf HJ, Specker BL (1995) Bone mineral loss during lactation and recovery after weaning. Obstet Gynecol 86(1):26–32
Lissner L, Bengtsson C, Hansson T (1991) Bone mineral content in relation to lactation history in pre- and postmenopausal women. Calcif Tissue Int 48:319
Lopez JM, Gonzalez G, Reyes V, Compino C, Dias S (1996) Bone turnover and density in healthy women during breastfeeding and after weaning. Osteoporo Int 6:153
Sowers MF, Corton G, Shapiro B et al (1993) Changes in bone density with lactation. JAMA 269:3130
Affinito P, Tommaselli GA, Di Carlo C, Guida F, Nappi C (1996) Changes in bone mineral density and calcium metabolism in breastfeeding women: a one year follow-up study. J Clin Endocrinol Metab 81:2314
Krebs NF, Reidinger CJ, Robertson AD, Brenner M (1997) Bone mineral density changes during lactation: maternal dietary, and biochemical correlates. Am J Clin Nutr 65:1738
Costa ML, Krupa FG, Rehder PM, Sousa MH, Costa-Paiva L, Cecatti JG (2012) Forearm bone mineral density changes during postpartum and the effects of breastfeeding, amenorrhea, body mass index and contraceptive use. Osteoporos Int 23(6):1691–1698
Aguado F, Revilla M, Hernandez ER, Menendez M, Cortes-Prieto J, Villa LF, Rico H (1998) Ultrasonographic bone velocity in pregnancy: a longitudinal study. Am J Obstet Gynecol 178:1016–1021
Hellmeyer L, Ossendorf A, Ziller V, Tekesin I, Schmidt S, Hadji P (2006) Quantitative ultrasonometry of the phalanges during pregnancy: a longitudinal study. Climacteric 9:446–451
Wüster C, Albanese C, De Aloysio D et al (2000) For the Phalangeal Osteosonogrammetry Study Group. Age-related changes, diagnostic sensitivity and discrimination power. J Bone Miner Res 15:1603–1614
Glüer CC (1997) Quantitative ultrasound technique for the assessment of osteoporosis: expert agreement on current status. J Bone Miner Res 12:1280–1288
Hans D, Dargent-Molina P, Schott AM et al (1996) Osteosonographic heel measurements to predict hip fracture in elderly women: the EPIDOS prospective study. Lancet 348:511–514
Yamaga A, Taga M, Minaguchi H, Sato K (1996) Changes in bone mass as determined by ultrasound and biochemical markers of bone turnover during pregnancy and puerperium: a longitudinal study. J Clin Endocrinol Metab 81(2):752–756
Hellmeyer L, Ziller V, Anderer G, Ossendorf A, Schmidt S, Hadji P (2006) Biochemical markers of bone turnover during pregnancy: a longitudinal study. Exp Clin Endocrinol Diabetes 114:506–510
Hadji P, Ziller V, Kalder M, Gottschalk M, Hellmeyer H, Hars O, Schmidt S, Schulz KD (2002) Influence of pregnancy and breast-feeding on quantitative ultrasonometry of bone in postmenopausal women. Climacteric 5:277–285
Okyay DO, Okyay E, Dogan E, Kurtulmus S, Acet F, Eftal Taner C. (2013) Prolonged breast-feeding is an independent risk factor for postmenopausal osteoporosis. Maturitas; 24
Tsvetov G, Levy S, Benbassat C, Shraga-Slutzky I, Hirsch D (2014) Influence of number of deliveries and total breast-feeding time on bone mineral density in premenopausal and young postmenopausal women. Maturitas 77(3):249–254
Hellmeyer L, Kühnert M, Ziller V, Schmidt S, Hadji P (2007) The use of i.v. bisphosphonate in pregnancy-associated osteoporosis—case study. Exp Clin Endocrinol Diabetes 115(2):139–142
Hellmeyer L, Boekhoff J, Hadji P (2010) Treatment with teriparatide in a patient with pregnancy-associated osteoporosis. Gynecol Endocrinol 26(10):725–728
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hellmeyer, L., Hahn, B., Fischer, C. et al. Quantitative ultrasonometry during pregnancy and lactation: a longitudinal study. Osteoporos Int 26, 1147–1154 (2015). https://doi.org/10.1007/s00198-014-2984-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-014-2984-y