Abstract
Summary
In a population-based study on cobalamin status and incident fractures in elderly men (n = 790) with an average follow-up of 5.9 years, we found that low levels of metabolically active and total cobalamins predict incident fractures, independently of body mass index (BMI), bone mineral density (BMD), plasma total homocysteine (tHcy), and cystatin C.
Introduction
Cobalamin deficiency in elderlies may affect bone metabolism. This study aims to determine whether serum cobalamins or holotranscobalamin (holoTC; the metabolic active cobalamin) predict incident fractures in old men.
Methods
Men participating in the Gothenburg part of the population-based Osteoporotic Fractures in Men (MrOS) Sweden cohort and without ongoing vitamin B medication were included in the present study (n = 790; age range, 70–81 years).
Results
During an average follow-up of 5.9 years, 110 men sustained X-ray-verified fractures including 45 men with clinical vertebral fractures. The risk of fracture (adjusted for age, smoking, BMI, BMD, falls, prevalent fracture, tHcy, cystatin C, 25-OH-vitamin D, intake of calcium, and physical activity (fully adjusted)), increased per each standard deviation decrease in cobalamins (hazard ratio (HR), 1.38; 95 % confidence intervals (CI), 1.11–1.72) and holoTC (HR, 1.26; 95 % CI, 1.03–1.54), respectively. Men in the lowest quartile of cobalamins and holoTC (fully adjusted) had an increased risk of all fracture (cobalamins, HR = 1.67 (95 % CI, 1.06–2.62); holoTC, HR = 1.74 (95 % CI, 1.12–2.69)) compared with quartiles 2–4. No associations between folate or tHcy and incident fractures were seen.
Conclusions
We present novel data showing that low levels of holoTC and cobalamins predicting incident fracture in elderly men. This association remained after adjustment for BMI, BMD, tHcy, and cystatin C. However, any causal relationship between low cobalamin status and fractures should be explored in a prospective treatment study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is one of the most common age-related diseases and the identification of causative, not least modifiable, risk factors is important. Subclinical deficiency of cobalamin (vitamin B12) is common in the elderly [1], mainly because of malabsorption caused by atrophic gastritis [2], which in turn has been suggested to have a negative effect on bone metabolism [3]. Similarly, cobalamin deficiency caused by autoimmune atrophic gastritis, has been identified as a risk factor for osteoporosis [4]. Bone mineral density (BMD) and risk of fractures in relation to serum cobalamins, folate, and plasma total homocysteine (tHcy) has been studied in both cross-sectional and prospective studies, but the results have been inconsistent [5–9]. It is unclear whether intervention with B vitamins can prevent fractures, but studies are ongoing [10]. In a placebo-controlled study of stroke patients [11], fracture incidence was reduced after cobalamin and folate supplementation; however, other studies have not been able to demonstrate efficacy [12].
Serum concentrations of cobalamins have low sensitivity and specificity for diagnosing subclinical deficiency [13]. tHcy reflects the intracellular availability of cobalamins, folate, and vitamin B6. However, tHcy accumulates also in renal insufficiency and further correlates to glomerular filtration rate (GFR) within the normal range [14]. Thus, tHcy is an unspecific marker for cobalamin deficiency, and its strong dependence on GFR has implications for the utility in elderly populations. On average, only 30 % of cobalamins are bound to transcobalamin (i.e., holotranscobalamin (holoTC)) and thus available for cellular uptake [15, 16]. HoloTC represents the functionally important fraction of cobalamins and is suggested the most sensitive marker for early cobalamin deficiency [17]. The association between holoTC and BMD/fractures, has to our knowledge, not been studied.
The aim of the present study was to investigate the predictive role of cobalamins, both measured as serum cobalamin and holoTC, for incident fracture in a large, prospectively followed up cohort of elderly men.
Materials and methods
Participants
Osteoporotic fractures in men (MrOS) is an epidemiological investigation of elderly men. It is an international, multicentre, prospective study focused on bone metabolism, and details regarding the Swedish cohort have been published previously [18]. For the present study, the Gothenburg MrOS site, subjects (n = 1010) were systematically selected from national population registries and invited to participate. To qualify for the study, men had to be able to walk unassisted and provide self-reported data about medical history including current medication and lifestyle characteristics. Written informed consent was obtained from all participants. All subjects were interviewed by questionnaire on previous fractures after 50 years of age, any falls during the previous 12 months, ongoing major disease, smoking, and other significant lifestyle factors including alcohol intake. Dietary calcium intake (milligrams per day) was assessed by diet questionnaires. Ongoing medication was assessed by interview and the probands' delivery of ongoing medication including both prescribed and over-the-counter drugs. All medication was registered according to the classification of the Swedish pharmacological registry. For the present study, a total of 220 (22 %) subjects were excluded because of ongoing medication with B vitamin supplements (n = 108) or pharmacological doses of vitamin B12, folic acid and/or vitamin B6 (n = 99) or combination of both (n = 13). The remaining subjects (n = 790) thus formed the total study group (TSG). The median age was 75.3 (range, 70.5–81.0) years. The study was approved by the ethics committee at the University of Gothenburg (M 014–01) and conducted in accordance with the guidelines in The Declaration of Helsinki.
Dual X-ray absorptiometry
BMD of the total hip including femoral trochanter and femoral neck, and in addition lumbar spine (vertebrae L1–4), total fat mass, and total lean mass, was measured by dual-energy X-ray absorptiometry using a Hologic QDR 4500/A-Delphi equipment (Hologic, Waltman, MA, USA). The coefficient of variation for this method (BMD expressed as grams per square centimeter) ranged from 0.5 to 3 %.
Assessment of covariates
Levels of habitual physical activity were quantified using parts of the questions in the Physical Activity Scale for Elderly [19]. Physical performance was evaluated with a 6-m walking test (n = 760), time stand test (n = 749), handgrip strength test (n = 755), and a balance test (n = 388) [20]. Body mass index (BMI) was calculated as weight in kilograms divided by height in square meters squared.
Assessment of incident fractures
Participants were followed up for a mean of 5.9 years (range, 4.7–7.4) after the baseline examination [18]. Time to first fracture or death was defined as time from the baseline study date to the actual event. Fracture evaluation during follow-up was in addition done by re-evaluation of X-ray in the regional registry, identified by the probands' unique personal registration number. Fractures rates were expressed as the number of subjects with first fractures per 1,000 person-years (Table 1). The total number of incident fractures was defined as all fractures comprising the following types: clinical vertebral osteoporosis fractures, nonvertebral osteoporosis fractures, and other fractures. Clinical vertebral fractures (clinical symptoms reported by the participants after the baseline visit) were confirmed by physician review of radiology report. Fractures reported by the study participants but not possible to confirm by radiology report were not included in the analysis. “Nonvertebral osteoporosis fractures” included hip, distal radius, proximal humerus, and pelvis, i.e., the major locations for osteoporotic fracture. “Other fractures” included radius/ulna, hand, fingers, humerus, elbow, skull, cervical vertebrae, clavicle, scapula, rib, femoral shaft, patella, upper tibia, ankle, foot, and toes. Dates for any such additional incident fracture in single subjects were recorded separately [21].
Blood sampling and analytical methods
All plasma/serum samples were collected at 8:00 a.m. after at least 10 h of fasting and nonsmoking. Samples were frozen immediately and stored at −80 °C. HoloTC was determined by an automatic method, AxSYM® HoloTC (Abbott), a microparticle enzyme immunoassay [22]. Cutoff limit for low values, as suggested by the manufacturer, is 19 pmol/L [23]. The total imprecision was 3.5 % at a concentration of 75 pmol/L. Cobalamins (pmol/L) were analyzed using electrochemiluminescence immunoassay on the Cobas Modular (Roche Diagnostics). The total imprecision was 4 % at a concentration of 220 pmol/L. Plasma tHcy (micromoles per liter) was measured on a Hitachi Modular-P (Roche). Cystatin C (milligrams per liter) was measured by immunoturbidimetry using Hitachi Modular-P analysers (Daco A/S Copenhagen). The total imprecision was 2.1 %. Methods for serum 25(OH) D, plasma total osteocalcin, parathyroid hormone levels, and serum levels of the N-terminal propeptide of type 1 procollagen (PINP) have been described previously [24, 25].
Statistical analyses
Standard methods were used for tests of correlation and group difference. The Pearson correlation was used for unadjusted tests of correlations and the Welch–Satterthwaite t test for test of differences in mean between two groups. Linear regression models were used for tests of correlation and group difference adjusted for potential confounding factors. The distribution of most of the continuous blood measurements showed considerable skewness and were analyzed after logarithmic transformation. One outlier value of holoTC, 1,783, was assigned the value of 950 before taking the log. The data from this single subject did not significantly influence the overall results. To explore the association between cobalamins, holoTC, and prospective risk of fractures, Poisson regression models or Cox proportional hazards models were used to estimate the hazard function. Spline functions were used in the risk models to achieve a flexible description of the association not assuming a constant hazard ratio over the entire range of the risk factors. The spline Poisson regression model was fitted using knots at the 10th, 50th, and 90th percentiles, as recommended by Harrell [26], of cobalamins and holoTC to study the association between these variables and fracture risk in more detail. The splines were second-order functions between the breakpoints and linear functions at the tails resulting in a smooth curve. Adjustment factors in the risk models included age, time since baseline, BMI, and BMD and were chosen as they were considered among the most significant markers for fracture. Adjusted hazard ratios (HR) per standard deviation (SD) are given with 95 % confidence intervals (CIs) within parentheses. Double-sided tests were used throughout, and a significance level of p < 0.05 was regarded as statistically significant. Software used were a combination of SAS for Windows, v9.2, SPSS for Windows v17, and a statistical program package developed at the Community Medicine and Public Health, University of Gothenburg.
Baseline characteristics of the study subjects
The general characteristics of participants in the study are presented in Table 1.
Results
Cobalamins, holoTC, and fracture risk
The number and incidence/1,000 person-years of fractures are shown in Table 1. In total, 110 subjects had at least one fracture. The group with any incident fracture (n = 110) as well as the subgroup with clinical vertebral fractures (n = 45) had significantly lower mean cobalamins, 311.1 and 278.6 pmol/L, as compared with the remaining nonfracture subjects, 346.9 and 345.6 pmol/L. In age-adjusted Cox's proportional hazard models, cobalamins was inversely related to the risk of first fracture (HR, 1.37; 95 % CI, 1.13–1.66/SD decrease) (Table 2). Subanalyses of fracture types showed that cobalamins were inversely related to risk of both clinical vertebral and other fractures (all validated fractures minus nonvertebral osteoporosis fractures and clinical vertebral fractures) with HR of 1.62 (95 % CI, 1.21–2.16) and 1.64 (95 % CI, 1.23–2.18) increased risk per SD decrease in cobalamins. After adjustment for confounders that could influence either risk of fracture or cobalamins (BMI, BMD, falls, prevalent fracture, tHcy, cystatin C, vitamin D, intake of calcium, smoking, and physical activity), this inverse association was still significant (Table 2). There were no statistically significant correlations (age adjusted) between cobalamins and hip fracture (HR, 1.50; 95 % CI, 0.96–2.34) or nonvertebral fractures (defined as distal radius, hip, proximal humerus, and pelvis; HR, 1.24; 95 % CI, 0.90–1.72). The group with any incident fracture (n = 110) as well as the subgroup with clinical vertebral fracture(s) (n = 45) had significantly lower mean holoTC (56.9 and 45.0 pmol/L) than the remaining nonfracture subjects (63.2 and 63.3 pmol/L). Age-adjusted Cox's proportional hazard models demonstrated that holoTC was inversely related to the risk of first fracture with HR of 1.27 (95 % CI, 1.05–1.54/SD decrease) (Table 2). Subanalyses of fracture types demonstrated that holoTC was inversely related to risk of clinical vertebral fractures with HR of 1.65 (95 % CI, 1.25–2.19) increased risk per SD decrease in holoTC. Additional adjustment for BMI, BMD, falls, prevalent fracture, tHcy, cystatin C, vitamin D, intake of calcium, smoking, and physical activity only slightly affected this inverse association (Table 2). Further adjustment for proton pump inhibitors use and alcohol intake did not change the results for either cobalamins or holoTC. HoloTC was not statistically significantly related to risk of hip fractures (HR, 1.29; 95 % CI, 0.85–1.95), nonvertebral fractures (HR, 1.01; 95 % CI, 0.73–1.40) or other fractures (HR, 1.22; 95 % CI, 0.92–1.62).
Quartiles of cobalamins and holoTC are presented in Table 3. Men in the lowest quartile of cobalamins and holoTC had, adjusted for age, an increased risk of all fractures (HR, 1.81 (95 % CI, 1.21–2.72/SD decrease) and 1.89 (95 % CI, 1.26–2.84 per SD decrease)) compared with quartiles 2–4. Corresponding figures for vertebral fracture were HR at 2.14 (95 % CI, 1.13–4.05) and 2.58 (95 % CI, 1.39–4.80). Further adjustment for BMD did not have a major impact on the results (Table 4). The results were somewhat attenuated when further adjustment for BMI, falls, prevalent fracture, tHcy, cystatin C, vitamin D, intake of calcium, smoking, and physical activity were performed, and the association between cobalamins and clinical vertebral fractures did not reach statistical significance (Table 4).
Spline models of the association between cobalamins, holoTC, and risk of fractures
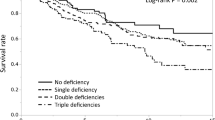
Further evaluation of the association between cobalamins, holoTC and yearly incidence of fractures using spline models adjusted for age, BMI, BMD, cystatin C, and tHcy was performed (Fig. 1a, b). This demonstrated that both cobalamins and holoTC below the median (310 and 51.8 pmol/L) was inversely related to incidence of fractures. The highest risk of fracture was seen for the 10 % of the subjects with cobalamins of <200 pmol/L and holoTC of <29.1 pmol/L.
a The hazard function of fracture (momentary risk) and 95 % confidence intervals according to cobalamin for a man aged 75 years after 2 years of follow-up. BMI, BMD, tHcy, and cystatin C is set to average value of the cohort. The vertical lines represent the 10th, 25th, 50th, and the 90th percentiles. b The hazard function of fracture (momentary risk) and 95 % confidence intervals according to holoTC for a man aged 75 years after 2 years of follow-up. BMI, BMD, tHcy, and cystatin C is set to average value of the cohort. The vertical lines represent the 10th, 25th, 50th, and the 90th percentiles
Cobalamins, holoTC in relation to Hb, iron status, and renal function
The proportion in the TSG of subjects with “low” serum concentrations of cobalamins (<140 pmol/L) were 1.7 and 25.6 % (<258 pmol/L) [27]. HoloTC of <19.6 pmol/L were seen in 3.1 % [28].
HoloTC correlated with cobalamins (r = 0.69, p < 0.001) and folate (r = 0.19, p < 0.001) and was negatively correlated with tHcy (r = −0.24, p < 0.001) but no correlations with hemoglobin (Hb), erythrocyte mean corpuscular volume, cystatin C, serum iron, ferritin, or transferrin saturation were seen [28]. Age-adjusted partial correlations between cobalamins/holoTC, folate, tHcy, and biochemistry/BMD/physical performance are shown in Table 5.
Vitamin B status in relation to BMD, falls, lifestyle, and physical performance ability
There were no partial correlations between cobalamins, holoTC, and BMD in age-adjusted models. In age-adjusted linear regression analysis, quartiles 1 and 2 of holoTC were associated with lower lumbar spine L1–4 BMD compared to quartiles 3 and 4 (β = −0.03, p = 0.028), but no associations with the hip sites were seen. This correlation was significant even after stepwise adjustment for smoking, BMI, falls, prevalent fracture, tHcy, cystatin C, 25-OH-vitamin D, intake of calcium, and physical activity (data not shown). There were no such associations between cobalamins and BMD.
Of 14 % (108/769 men), reported at least one fall during the year preceding the baseline visit. There were no differences in mean cobalamins (p = 0.10), holoTC (p = 0.08), folate (p = 0.22), or tHcy (p = 0.55) between fallers and nonfallers in age-adjusted regression analysis. Both holoTC and cobalamin correlated positively with intake of calcium (r = 0.18, p < 0.001 and r = 0.21, p < 0.001, respectively). There were few and weak associations between vitamin B status and lifestyle factors/physical performance as shown in Table 5. Subjects with serum cobalamin of <200 pmol/L had significantly lower intake of calcium compared with subjects with cobalamin of >200 pmol/L (793.7 vs 929.2 mg/day, p < 0.05), but there were no differences between the two groups regarding age, renal function, previous fracture, falls, smoking habits, prevalence of diabetes, stroke, myocardial infarction, or cancer (data not shown).
Vitamin B substitution and incident fractures
To evaluate any correlation between B vitamin substitution and fracture incidence, subjects with ongoing medication with B vitamin supplements (n = 108) or pharmacological doses of vitamin B12, folic acid and/or vitamin B6 (n = 99) or combination of both (n = 13) at start of the study (n = 220; hereinafter described as vitamin B substituted subjects) were included in addition to the TSG (n = 790).
Vitamin B substituted subjects were older (75.9 vs 75.1 years, p < 0.01), had lower BMI (25.8 vs 26.3 kg/m2, p < 0.05), Hb (143.6 vs 147.5 g/L, p < 0.001), tHcy (11.8 vs 15.0 μmol/L, p < 0.001), and higher cystatin C (1.16 vs 1.12 mg/L, p < 0.05) compared with nonvitamin B-substituted subjects (n = 790).
This population was then divided into three groups; subjects with holoTC of <38.9 pmol/L (the lowest quartile of holoTC, n = 195), subjects with holoTC of ≥38.9 pmol/L (quartiles 2–4, n = 586) and vitamin B substituted subjects (n = 220). The fracture incidence during follow-up in these three groups were 20 % (39/195), 11.8 % (69/586), and 19.1 % (42/220), respectively. Subjects with the lowest incidence of all fractures (quartiles 2–4 of holoTC) were used as reference group. Vitamin B-substituted subjects had an increased risk of fracture (HR, 1.70; 95 % CI, 1.14–2.52), age adjusted, compared with the reference group. However, when adjusted for age, smoking, BMI, BMD, falls, prevalent fracture, tHcy, cystatin C, 25-OH-vitamin D, intake of calcium, and physical activity, the increased risk of fracture for vitamin B substituted subjects was no longer significant (HR, 1.40; 95 % CI, 0.91–2.15).
Folate, tHcy, and fracture risk
No significant differences in mean tHcy or folate between fracture and nonfracture subjects were observed (data not shown). There were no correlations between folate, tHcy, and incident fracture either for overall fractures (HR, 1.17 (95 % CI, 0.97–1.40) and 1.0 (95 % CI, 0.83–1.21)) or as analyzed by fracture subtype.
Discussion
In this study of community-dwelling elderly men, low concentrations of cobalamins and holoTC correlated independently to increased risk of fracture, after adjustment for possible covariates, such as smoking, BMI, tHcy, renal function, BMD, previous fractures, falls, physical performance, serum 25(OH)D, and intake of calcium. Decision limits for cobalamin deficiency as defined by total serum cobalamins are not easily defined. There is a large grey zone, ranging from about 140 to 258 pmol/L [27]. When using these limits for total serum cobalamins in our cohort, the prevalence of cobalamin “deficiency” ranged from 2 to 25 %.
However, we also used plasma holoTC, which is considered the most sensitive marker for early cobalamin depletion [29]. It is noteworthy that the “lowest effective” cobalamin levels to ameliorate fracture risk as interpreted from the spline regression models of our data corresponds to the median values for cobalamin and holoTC concentrations calculated for this essentially “nondeficient” population. Our results point to correlations between cobalamin status and fractures in the low but traditionally “noncobalamin-deficient” range, which to our knowledge is a novel finding. This might indicate that bone tissue actually requires higher plasma levels of cobalamins for normal homeostasis than, e.g., for hematopoiesis.
The pathophysiological mechanism(s) by which insufficient cobalamin status might affect normal bone metabolism are yet to be elucidated. We found no correlations between cobalamin/holoTC and the bone formation markers in agreement with some [30] but not with others [3, 31]. In vitro studies using receptor activator of NF-kB ligand (RANKL)-stimulated human peripheral blood monocytes cultured on dentine slices have shown that decreasing concentrations of cobalamins results in increased formation of resorption pits [32]. It was not investigated if this was due to increased osteoclast formation or bone resorbing activity. A study using mouse bone marrow macrophages reported that cobalamins did not affect RANKL-stimulated osteoclast differentiation [33], indicating that cobalamins might influence osteoclast acitivity rather than formation. Cobalamins seem to have no effect on osteoblastic differentiation of multipotent human bone marrow stromal cells [33] or on the bone-forming activity of human osteoblasts isolated from trabecular bone [34].
Studies on the association between cobalamins and BMD (as well as tHcy-BMD) have shown diverging results [5, 35–37], possibly due to varying degrees of cobalamin depletion in the populations investigated and the poor specificity of cobalamins as a measure of cobalamin depletion. In the present study, quartiles 1 and 2 of holoTC (but not cobalamins) were associated with lower lumbar spine BMD and holoTC was related to incident clinical vertebral fractures. We did only find associations between holoTC and cobalamins and on the other hand all fractures (n = 110), vertebral fractures (n = 45), and for cobalamins also other fractures (n = 49), but not at sites typically associated with osteoporotic fractures like nonvertebral osteoporotic fractures (n = 36) including hip fractures (n = 17). In nonvitamin B-treated subjects, cobalamins were further positively correlated with serum 25-OH-vitamin D and intake of calcium, holoTC with the latter, indicating an insufficient diet with respect to bone health. Falling is a well-known risk factor for fracture. Cobalamin deficiency is associated with peripheral neuropathy and poor locomotor function [38]. Thus, low cobalamin stores might cause increased fractures by increasing the risk of falling. However, neither cobalamins nor tHcy were associated with falls, in accordance with other studies [31], and we found very few correlations between cobalamins and variables of physical performance.
Subjects with ongoing medication with B vitamin supplements or pharmacological doses of vitamin B12, folic acid, and/or vitamin B6 (n = 220) at the start of the study had a higher risk of incident fractures compared with subjects with holoTC of ≥38.9 pmol/L(quartiles 2–4 of holoTC) and no B vitamin substitution. However, vitamin B-substituted subjects were older, had lower BMI and Hb levels, and higher cystatin C, i.e., factors that reflect morbidity, furthermore, when fully adjusted, the increased risk for fracture was attenuated and no longer significant. We cannot from these retrospective data conclude whether cobalamin treatment per se is beneficial or not. In a French study of 1,482 older men and women, higher intake of cobalamin was related to a lower risk of wrist and hip fracture [39] and fracture incidence was reduced in stroke patients after cobalamin and folate treatment [11]. Notably, cystatin C has recently been shown as an independent risk factor for hip fracture in women [40] and as the vitamin B-substituted subjects (n = 220) had a slightly worse renal function compared with nonvitamin B-substituted subjects (n = 790), this might have contributed to the increased risk of fracture in this group.
We did not find any association between tHcy and risk of fracture, in contrast with some other studies [6, 7, 9, 41, 42]. However, in the Framingham cohort [9], when adjusting for cobalamin, this association was no longer significant. Furthermore, in this study low concentrations of cobalamins (<148 pmol/L) were associated with increased risk of hip fracture, however when adjusting for BMD and tHcy, no statistical significance was seen [9].
As discussed above, reduced renal function increases the risk of fracture [43, 44] and the effect of renal function on tHcy levels has been proposed to contribute to this association [42]. Our data do not support an association between tHcy and fracture risk, but rather a relationship between cobalamins and fracture, further supported by a connection between low holoTC and BMD.
Our study might have several limitations. First, this study was restricted to Swedish men and thus possibly not generalizable to other ethnic groups or women. Second, the men included in this study had to be able to walk without aid and therefore possibly representing a healthier subgroup than the general population. This might have contributed to the fact that we did not find any correlations between physical performance and cobalamin status.
A further limitation is the cross-sectional character of the study with single measurement of cobalamins and holoTC. An additional limitation was that trauma severity was not taken into account in fracture ascertainment, even though falls were registered and adjusted for in various analyses. The strength of our study is the prospective evaluation of incident fractures in a relatively large population, considering relevant confounding factors, such as BMD, cystatin C, and tHcy.
In conclusion, we found, in nonvitamin B-treated elderly men, a correlation between cobalamin status, as measured with both cobalamins and holoTC, and increased fracture risk, independent of other established risk factors for osteoporosis. The mechanism(s) by which cobalamin interacts with bone tissue needs to be further elucidated. Whether “low” cobalamin status represents a modifiable risk factor for osteoporotic fractures in the elderly should be the subject of future intervention studies.
References
Loikas S, Koskinen P, Irjala K, Lopponen M, Isoaho R, Kivela SL, Pelliniemi TT (2007) Vitamin B12 deficiency in the aged: a population-based study. Age Ageing 36:177–183
Lewerin C, Jacobsson S, Lindstedt G, Nilsson-Ehle H (2008) Serum biomarkers for atrophic gastritis and antibodies against Helicobacter pylori in the elderly: implications for vitamin B12, folic acid and iron status and response to oral vitamin therapy. Scand J Gastroenterol 43:1050–1056
Carmel R, Lau KH, Baylink DJ, Saxena S, Singer FR (1988) Cobalamin and osteoblast-specific proteins. N Engl J Med 319:70–75
Espallargues M, Sampietro-Colom L, Estrada MD, Sola M, del Rio L, Setoain J, Granados A (2001) Identifying bone-mass-related risk factors for fracture to guide bone densitometry measurements: a systematic review of the literature. Osteoporos Int 12:811–822
Herrmann M, Peter Schmidt J, Umanskaya N, Wagner A, Taban-Shomal O, Widmann T, Colaianni G, Wildemann B, Herrmann W (2007) The role of hyperhomocysteinemia as well as folate, vitamin B(6) and B(12) deficiencies in osteoporosis: a systematic review. Clin Chem Lab Med 45:1621–1632
McLean RR, Jacques PF, Selhub J, Tucker KL, Samelson EJ, Broe KE, Hannan MT, Cupples LA, Kiel DP (2004) Homocysteine as a predictive factor for hip fracture in older persons. N Engl J Med 350:2042–2049
van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM et al (2004) Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med 350:2033–2041
Yazdanpanah N, Zillikens MC, Rivadeneira F, de Jong R, Lindemans J, Uitterlinden AG, Pols HA, van Meurs JB (2007) Effect of dietary B vitamins on BMD and risk of fracture in elderly men and women: the Rotterdam study. Bone 41:987–994
McLean RR, Jacques PF, Selhub J, Fredman L, Tucker KL, Samelson EJ, Kiel DP, Cupples LA, Hannan MT (2008) Plasma B vitamins, homocysteine, and their relation with bone loss and hip fracture in elderly men and women. J Clin Endocrinol Metab 93:2206–2212
van Wijngaarden JP, Dhonukshe-Rutten RA, van Schoor NM et al (2011) Rationale and design of the B-PROOF study, a randomized controlled trial on the effect of supplemental intake of vitamin B12 and folic acid on fracture incidence. BMC Geriatr 11:80
Sato Y, Honda Y, Iwamoto J, Kanoko T, Satoh K (2005) Effect of folate and mecobalamin on hip fractures in patients with stroke: a randomized controlled trial. JAMA 293:1082–1088
Sawka AM, Ray JG, Yi Q, Josse RG, Lonn E (2007) Randomized clinical trial of homocysteine level lowering therapy and fractures. Arch Intern Med 167:2136–2139
Carmel R (2011) Biomarkers of cobalamin (vitamin B-12) status in the epidemiologic setting: a critical overview of context, applications, and performance characteristics of cobalamin, methylmalonic acid, and holotranscobalamin II. Am J Clin Nutr 94:348S–358S
Lewerin C, Ljungman S, Nilsson-Ehle H (2007) Glomerular filtration rate as measured by serum cystatin C is an important determinant of plasma homocysteine and serum methylmalonic acid in the elderly. J Intern Med 261:65–73
Quadros EV (2010) Advances in the understanding of cobalamin assimilation and metabolism. Br J Haematol 148:195–204
Ulleland M, Eilertsen I, Quadros EV, Rothenberg SP, Fedosov SN, Sundrehagen E, Orning L (2002) Direct assay for cobalamin bound to transcobalamin (holo-transcobalamin) in serum. Clin Chem 48:526–532
Herrmann W, Obeid R, Schorr H, Geisel J (2003) Functional vitamin B12 deficiency and determination of holotranscobalamin in populations at risk. Clin Chem Lab Med 41:1478–1488
Mellstrom D, Johnell O, Ljunggren O, Eriksson AL, Lorentzon M, Mallmin H, Holmberg A, Redlund-Johnell I, Orwoll E, Ohlsson C (2006) Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res 21:529–535
Washburn RA, Smith KW, Jette AM, Janney CA (1993) The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46:153–162
Ribom EL, Grundberg E, Mallmin H, Ohlsson C, Lorenzon M, Orwoll E, Holmberg AH, Mellstrom D, Ljunggren O, Karlsson MK (2009) Estimation of physical performance and measurements of habitual physical activity may capture men with high risk to fall—data from the Mr Os Sweden cohort. Arch Gerontol Geriatr 49:e72–e76
Mellstrom D, Vandenput L, Mallmin H et al (2008) Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J Bone Miner Res 23:1552–1560
Orning L, Rian A, Campbell A, Brady J, Fedosov SN, Bramlage B, Thompson K, Quadros EV (2006) Characterization of a monoclonal antibody with specificity for holo-transcobalamin. Nutr Metab Lond 3:3
Brady J, Wilson L, McGregor L, Valente E, Orning L (2008) Active B12: a rapid, automated assay for holotranscobalamin on the Abbott AxSYM analyzer. Clin Chem 54:567–573
Johansson H, Oden A, Lerner UH, et al. (2012) High serum adiponectin predicts incident fractures in elderly men. Mr OS Sweden. J Bone Miner Res 27:1390–1396
Kindblom JM, Ohlsson C, Ljunggren O, Karlsson MK, Tivesten A, Smith U, Mellstrom D (2009) Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res 24:785–791
Harrell FE (ed) (2001) General aspects of fitting regression models: regression modeling strategies. Springer, New York
Lindenbaum J, Rosenberg IH, Wilson PW, Stabler SP, Allen RH (1994) Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr 60:2–11
Lewerin C, Nilsson-Ehle H, Jacobsson S, Karlsson MK, Ohlsson C, Mellstrom D (2013) Holotranscobalamin is not influenced by decreased renal function in elderly men: the MrOS Sweden study. Ann Clin Biochem (in press)
Obeid R, Herrmann W (2007) Holotranscobalamin in laboratory diagnosis of cobalamin deficiency compared to total cobalamin and methylmalonic acid. Clin Chem Lab Med 45:1746–1750
Nilsson K, Gustafson L, Isaksson A, Hultberg B (2005) Plasma homocysteine and markers of bone metabolism in psychogeriatric patients. Scand J Clin Lab Invest 65:671–680
Dhonukshe-Rutten RA, Pluijm SM, de Groot LC, Lips P, Smit JH, van Staveren WA (2005) Homocysteine and vitamin B12 status relate to bone turnover markers, broadband ultrasound attenuation, and fractures in healthy elderly people. J Bone Miner Res 20:921–929
Herrmann M, Schmidt J, Umanskaya N, Colaianni G, Al Marrawi F, Widmann T, Zallone A, Wildemann B, Herrmann W (2007) Stimulation of osteoclast activity by low B-vitamin concentrations. Bone 41:584–591
Vaes BL, Lute C, Blom HJ, Bravenboer N, de Vries TJ, Everts V, Dhonukshe-Rutten RA, Muller M, de Groot LC, Steegenga WT (2009) Vitamin B(12) deficiency stimulates osteoclastogenesis via increased homocysteine and methylmalonic acid. Calcif Tissue Int 84:413–422
Herrmann M, Umanskaya N, Wildemann B, Colaianni G, Schmidt J, Widmann T, Zallone A, Herrmann W (2007) Accumulation of homocysteine by decreasing concentrations of folate, vitamin B12 and B6 does not influence the activity of human osteoblasts in vitro. Clin Chim Acta 384:129–134
Morris MS, Jacques PF, Selhub J (2005) Relation between homocysteine and B-vitamin status indicators and bone mineral density in older Americans. Bone 37:234–242
Tucker KL, Hannan MT, Qiao N, Jacques PF, Selhub J, Cupples LA, Kiel DP (2005) Low plasma vitamin B12 is associated with lower BMD: the Framingham Osteoporosis Study. J Bone Miner Res 20:152–158
Gjesdal CG, Vollset SE, Ueland PM, Refsum H, Drevon CA, Gjessing HK, Tell GS (2006) Plasma total homocysteine level and bone mineral density: the Hordaland Homocysteine Study. Arch Intern Med 166:88–94
Lewerin C, Matousek M, Steen G, Johansson B, Steen B, Nilsson-Ehle H (2005) Significant correlations of plasma homocysteine and serum methylmalonic acid with movement and cognitive performance in elderly subjects but no improvement from short-term vitamin therapy: a placebo-controlled randomized study. Am J Clin Nutr 81:1155–1162
Samieri C, Ginder Coupez V, Lorrain S, Letenneur L, Alles B, Feart C, Paineau D, Barberger-Gateau P (2013) Nutrient patterns and risk of fracture in older subjects: results from the Three-City Study. Osteoporos Int 24:1295–1305
Ensrud KE, Parimi N, Cauley JA, Ishani A, Slinin Y, Hillier TA, Taylor BC, Steffes M, Cummings SR (2013) Cystatin C and risk of hip fractures in older women. J Bone Miner Res (in press)
Leboff MS, Narweker R, LaCroix A et al (2009) Homocysteine levels and risk of hip fracture in postmenopausal women. J Clin Endocrinol Metab 94:1207–1213
LaCroix AZ, Lee JS, Wu L et al (2008) Cystatin-C, renal function, and incidence of hip fracture in postmenopausal women. J Am Geriatr Soc 56:1434–1441
Ensrud KE, Lui LY, Taylor BC, Ishani A, Shlipak MG, Stone KL, Cauley JA, Jamal SA, Antoniucci DM, Cummings SR (2007) Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med 167:133–139
Fried LF, Biggs ML, Shlipak MG et al (2007) Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol 18:282–286
Acknowledgments
The research was supported by the Swedish Research Council, the Swedish Foundation for Strategic Research, and the ALF/LUA research grant in Gothenburg.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lewerin, C., Nilsson-Ehle, H., Jacobsson, S. et al. Low holotranscobalamin and cobalamins predict incident fractures in elderly men: the MrOS Sweden. Osteoporos Int 25, 131–140 (2014). https://doi.org/10.1007/s00198-013-2527-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-013-2527-y