Abstract
Summary
Data gathered from a nationally representative cohort demonstrate that higher serum ferritin levels are significantly associated with lower bone mass at various skeletal sites and the increased prevalence of osteoporosis and fractures, especially in women ≥45 years of age.
Introduction
Despite extensive in vitro and in vivo studies showing the detrimental effects of iron on bone metabolism, the clinical studies relating to osteoporosis-related phenotypes have not been evaluated extensively. In the present study, we investigated and compared the association between serum ferritin and bone mineral density (BMD), depending on the stratified age groups in both genders.
Methods
This is a population-based, cross-sectional study from the Korea National Health and Nutrition Examination Surveys, including 14,017 Koreans (6,817 men and 7,200 women) aged 10–80 years. BMD was measured using dual X-ray absorptiometry, and osteoporosis was diagnosed by the World Health Organization definition.
Results
Initially, we divided the subjects into three age groups, based on the patterns of age-related BMD changes in this national cohort (i.e., ≤24, 25–44, and ≥45 years old). Serum ferritin concentrations were inversely associated with BMD values at all measured sites after adjustment for confounders, only in women ≥45 years of age (P = 0.041 to <0.001). Furthermore, when we divided these women into serum ferritin quartiles, the odds for prevalent osteoporosis and fractures were 1.55-fold (95 % CI = 1.09–2.23) and 1.52-fold (95 % CI = 1.02–2.27) higher, respectively, in subjects in the highest quartile compared with those in the lowest quartile.
Conclusions
These results provide the first clinical evidence that the associations between serum ferritin level and bone parameters could be the most prominent in women ≥45 years of age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron is a transition metal that can catalyze the formation of hydroxyl radicals, which are powerful pro-oxidants that attack cellular membrane lipids, proteins, and nucleic acids, resulting in tissue damage [1, 2]. Therefore, increasing iron stores can contribute to the pathogenesis of various diseases, such as insulin resistance [3]. Many lines of evidence now indicate that iron has direct detrimental effects on bone metabolism as well. In vitro studies have shown that iron promotes osteoclast differentiation and bone-resorbing activity by enhancing mitochondrial biogenesis [4], whereas it suppresses osteoblastogenesis [5, 6]. Iron-overloaded mice demonstrate increased oxidative stress and bone resorption, leading to changes in bone microarchitecture and material properties and thus bone loss [7]. In addition, the increased prevalence of osteoporosis and fractures that are observed in patients with disorders associated with iron overload, such as hemochromatosis and thalassemia, independently of hypogonadism and cirrhosis, support these experimental and in vivo findings [8–10].
Despite these potential roles of iron in bone metabolism, clinical studies relating to osteoporosis-related phenotypes, especially in subjects with moderately elevated body iron stores that are below the levels commonly found in genetic hemochromatosis, have not been extensively evaluated. Only recently have we determined that body iron stores reflected by higher ferritin concentrations are significantly associated with accelerated bone loss at various proximal femur sites and a higher risk for incident radiologic vertebral fracture in the large longitudinal study of healthy Koreans [11]. This study has important implications in that it clinically validates previous experimental and animal data and provides the first clinical evidence that higher total body iron stores could be an independent risk factor for future deterioration of bone mass even under nonpathological conditions. However, the study population in the cited study was comprised of subjects who visited a health promotion center and may not have been representative of the general population, thus possibly resulting in selection bias.
Bone is a highly dynamic tissue that is constantly undergoing changes in response to biochemical and mechanical signals, and the characteristics of bone metabolism vary throughout life. By the removal of bone from one site and deposition at a different one during development and growth, the skeleton reaches peak bone mass; this process is called modeling. After achieving maturity, continuous remodeling occurs through a dynamic process of osteoclastic breakdown and osteoblastic rebuilding, which is normally balanced and tightly controlled by the coupling phenomenon [12]. Then, accelerated bone loss stage proceeds, especially in women predominantly after menopause. Therefore, the effects of risk factors on bone health could be various depending on biological differences in each stratified age group. In the present study, we investigated the association between serum ferritin concentrations and bone mineral density (BMD) at various skeletal sites using representative data from the general Korean population across a wide range of ages.
Materials and methods
Study population
We recruited participants from the second (2008) and third year (2009) of the fourth Korea National Health and Nutrition Examination Surveys (KNHANES) and the first year (2010) of the fifth KNHANES because BMD data have been available since 2008. This cross-sectional nationwide survey uses a stratified, multistage, clustered probability sampling method to select a representative sample of the noninstitutionalized, civilian Korean population [13]. The survey was composed of a health interview survey, a nutrition survey, and a health examination survey. Data were collected by household interviews and by direct standardized physical examinations that were conducted at mobile examination centers. All participants in this survey signed an informed consent form. The database of KNHANES is publicly available at the KNHANES website (http://knhanes.cdc.go.kr; available in Korean).
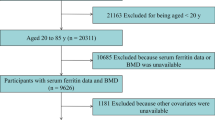
A total of 9,308, 10,078, and 8,473 subjects participated in KNHANES in 2008, 2009, and 2010, respectively, and the response rates were 74.3, 79.2, and 77.5 %, respectively. Among those who participated in the survey, BMD was measured in 17,965 subjects (7,903 men and 10,062 women) between the ages of 10 and 80 years from all 16 administrative districts of Korea. Initially, we excluded subjects who were missing data on serum ferritin (n = 838). Subjects with chronic liver diseases, chronic renal diseases, neoplastic diseases, increased serum liver enzyme activities [e.g., aspartate aminotransferase (AST) or alanine aminotransferase (ALT) >100 IU/L], increased serum creatinine levels (≥1.6 mg/dL), and/or abnormal leukocyte counts (>10,000 or <4,000 cells/mm3) were excluded from this study (n = 1,599). Subjects were also excluded if they had taken drugs for thyroid dysfunction and/or osteoporosis, such as bisphosphonate or estrogen (n = 1,434). Finally, subjects with exceptionally high serum ferritin levels (>500 ng/mL; n = 77) were excluded in order to rule out those who could potentially have hemochromatosis [14, 15]. The remaining 14,017 subjects (6,817 men and 7,200 women) were eligible for this study.
Lifestyle factors and anthropometric measurements
All subjects underwent a thorough physical examination. Age, body weight, height, smoking, drinking, and exercise habits, and calcium (Ca) and phosphorus (P) intake were recorded. Smoking habit was categorized into three levels (never, past, or current), and drinking habit was indicated as yes when the subject drinks alcohol above 3 U/day, respectively. Dietary intake of Ca and P were estimated using the 24-h dietary recall method. Exercise was indicated as yes when the subject exercised regularly at moderate levels (e.g., for more than 20 min per session and more than three times per week). Height (centimeters) and weight (kilograms) were measured using standardized protocols, while the subject was dressed in light clothing without shoes.
Biochemical measurements
Blood samples were obtained for biochemical analysis from all participants during the survey. These samples were immediately refrigerated, transported to the Central Testing Institute in Seoul, Korea, and then analyzed within 24 h. The serum ferritin level was determined using an immunoradiometric assay with a 1470 Wizard Gamma Counter (PerkinElmer, Turku, Finland; reference ranges = 30–400 ng/mL for men and 13–150 ng/mL for women). The coefficient of variation (CV) value was <5 %. The serum levels of iron and total iron binding capacity (TIBC) were measured by a bathophenanthroline direct method using a Hitachi automatic analyzer 7600 (Tokyo, Japan; reference ranges = 33–193 μg/dL and 266–422 μg/dL, respectively). Serum transferrin saturation was calculated from the serum levels of iron and TIBC {transferrin saturation (%) = [serum iron (μg/dL)/TIBC (μg/dL)] × 100} (reference range = 20–55 %). Serum 25-hydroxyvitamin D [25(OH)D] level was measured by a radioimmunoassay method using a 1470 Wizard Gamma Counter (sufficient level = 30 ng/mL or more). The serum levels of AST, ALT, and alkaline phosphatase (ALP) were measured enzymatically using a Hitachi automatic analyzer 7600 (reference ranges <40 IU/L for AST and ALT and 40–130 IU/L for ALP).
BMD measurements
Areal BMD (g/cm2) was measured using dual-energy X-ray absorptiometry (DXA; QDR 4500A, Hologic Inc., Waltham, MA, USA), which was performed at mobile examination centers and operated by licensed, trained technicians. The in vivo precision was 0.73–1.07 %, 1.20–2.14 %, and 0.71–1.18 % for the lumbar spine, femur neck, and total femur, respectively. These values were obtained by scanning randomly selected 30 subjects who underwent two scans on the same day, getting on and off the table between examinations. In the National Health and Nutrition Examination Survey (NHANES), the DXA instruments were calibrated using the methods in a previous report [16]. We obtained the reference values of the NHANES using this calibration method [17]. The NHANES calibrations were applied for appropriate comparisons between the present and previous data. We maintained DXA calibrations via an internal referencing system and daily measured spine phantoms, which are bone and soft tissue equivalent reference standards, during the examination, as previously described [18, 19]. BMD measurement provided absolute values for each anatomic site and, in postmenopausal women and/or women ≥50 years of age, were compared with those of healthy young Japanese adults (T-score), which were provided by the manufacturer of the bone densitometry equipment [13]. The mean reference BMD values at the lumbar spine, femur neck, and total femur in women are 1.006 ± 0.115, 0.803 ± 0.107, and 0.851 ± 0.115, respectively, whereas the values in men are 1.024 ± 0.120, 0.846 ± 0.124, and 0.940 ± 0.137 g/cm2, respectively. According to the World Health Organization (WHO), osteoporosis is diagnosed by a T-score ≤ −2.5 standard deviation (SD) at any of sites in the lumbar spine, femur neck, or total femur.
Fracture detection in personal histories
Fracture events were recorded using a standardized self-administered questionnaire. Fractures clearly caused by high-trauma events were excluded. High-trauma events included motor vehicle accidents, violence, and falls from more than the standing height of the individual. We included fracture events at only six sites (vertebra, hip, wrist, humerus, clavicle, and ribs). In addition, historical height loss, which was calculated as the difference between the subject’s current measured height and self-reported recalled tallest height, of 4.0 cm or more was regarded as indicative of a prevalent vertebral fracture [20–22].
Statistical analysis
The continuous and categorical variables are reported as the mean with 95 % confidence intervals (CIs) and percentages, respectively, unless otherwise specified. The baseline characteristics of the three groups were compared using one-way analysis of variance (ANOVA) for continuous variables or chi-square test for categorical variables. To determine the independent effects of serum iron-related markers, including ferritin, iron, TIBC, and transferrin saturation, on BMD at various skeletal sties, we used a multiple regression model with the BMD value at each skeletal site as the dependent variable and each serum iron-related marker as the independent variable. In these analyses, the serum ferritin concentration was logarithmically transformed because the distribution was positively skewed. Confounding independent variables were selected on the basis of clinical applicability. The base adjustment model included age, weight, and height. In addition to the factors included in the base model, the multivariable adjustment model included smoking, drinking, and exercise habits, serum 25(OH)D level, and Ca and P intake. To further test our hypothesis that higher ferritin levels may be associated with lower bone mass, especially in women ≥45 years of age, we categorized these women into four groups according to their serum ferritin concentrations and then performed the analyses. The multivariate-adjusted least-square mean (95 % CIs) BMD values, in terms of the serum ferritin quartiles, were estimated using analysis of covariance (ANCOVA) after adjustment for confounders. The trends in the BMD values at each skeletal site across increasing ferritin quartiles were checked by examining P value for trends using multiple linear regression analysis, with BMD values as the dependent variable and ordinal ferritin quartiles as the independent variable. ANCOVA was used to compare BMD values according to serum ferritin quartiles after adjustment for confounding variables. We performed multiple logistic regression analyses to generate odds ratios (ORs) that compared the odds of prevalent osteoporosis and fractures for subjects in each of the higher three ferritin quartiles to the odds of subjects in the lowest quartile after adopting the base and multivariable adjustment models. The independent association between serum ferritin and ALP levels was also investigated using a multiple regression analysis after adjustment for confounders. All analyses were performed by reflecting weighted values at the stratification of the samples applied to KNHANES. These statistical analyses were performed using SPSS statistical software (version 17.0; SPSS Inc., Chicago, IL, USA), and P < 0.05 was considered statistically significant.
Results
The mean ages of the 6,817 men and 7,200 women included in this study were 44.9 ± 18.5 (range, 10–93) and 43.8 ± 17.8 (range, 10–95) years, respectively. The baseline characteristics according to the age groups (i.e., ≤24 years old for age group I, 25–44 years old for age group II, and ≥45 years old for age group III) in each gender are shown in Table 1. Because the serum levels of iron, TIBC, and transferrin saturation were measured since 2010 in this national cohort, information about these variables was available for 2,621 (38.4 %) of the 6,817 men and 2,527 (35.1 %) of the 7,200 women and was used for analyses in Tables 1 and 2. Men in age group II and III demonstrated significantly higher serum levels of ferritin, iron, and transferrin saturation and lower serum level of TIBC than those in age group I. The markedly lower levels of serum ferritin in age group I may be mainly explained by the abrupt growth in a teenager and the resultant relative iron deficiency. The serum 25(OH)D level was the highest in men classified as in age group III; however, the differences were weak and seemed to have little clinical significance. The serum ALP level was the highest in men classified as in age group I because some of subjects in this age group may not complete their growth. Men in age group III had the lowest BMD values at the femur neck and total femur, whereas the lumbar spine BMD was the lowest in those in age group I. The consistent lower BMD values at all skeletal sites in men in age group III, compared with those in age group II, could be attributable to the higher mean ages and age-related biological changes in this group [23]. In women, subjects in age group III had the highest serum ferritin and the lowest serum TIBC levels, which could be favored by the cessation of menses. However, there were no significant differences in the serum levels of iron and transferrin saturation among those in three age groups. As observed in men, women in age group III had the highest serum 25(OH)D level, while those in age group I had the highest serum ALP level. Women in age group III consistently had the lowest BMD values at all measured skeletal sites.
Multiple regression analyses were used to examine the independent effects of the various iron-related markers on the BMD values at various skeletal sites in each stratified age group after considering other possible covariates (Table 2). In men, serum ferritin, iron, TIBC, and transferrin saturation did not contribute to the BMD values at any of the skeletal sites in any age groups. In women, serum iron-related markers had no significant association with the BMD values at any of the skeletal sites in age group I and II, except at the lumbar spine BMD in age group II, which demonstrated an inverse correlation with the serum ferritin concentration. On the other hand, the serum ferritin level was inversely associated with the BMD values at all measured skeletal sites in women in age group III after adopting the multivariable adjustment model. Therefore, we performed further analyses that focused on the effects of serum ferritin on bone metabolism in women ≥45 years of age, classified as age group III.
In women belonging to age group III, when we performed simple linear regression analyses with the BMD value at each skeletal site as the dependent variable and the log-transformed serum ferritin concentration as the independent variable, the coefficient of determination R 2 values at the lumbar spine, femur neck, and total femur were 0.072, 0.058, and 0.050, respectively. These results indicate that 7.2, 5.8, and 5.0 % of the variation in BMD at the lumbar spine, femur neck, and total femur, respectively, is explained by the serum ferritin concentration.
After women in age group III were categorized into the four groups according to their serum ferritin concentrations [1.1–28.0 ng/mL for Q1 (the lowest quartile), 28.1–50.5 ng/mL for Q2, 50.6–77.9 ng/mL for Q3, and 78.0–486.1 ng/mL for Q4 (the highest quartile)], multivariate-adjusted least-square mean BMD values were estimated after considering potential confounding factors (Fig. 1). The BMD value at the lumbar spine significantly decreased in a dose–response manner across increasing ferritin quartiles in both the base and multivariable adjustment models. Consistently, compared with women in Q1 in age group III, those in Q3 and Q4 demonstrated significantly lower lumbar spine BMD values. However, the trends for the BMD values that were determined at the femur neck and total femur, in terms of the serum ferritin quartiles, were not statistically significant.
Bone mineral density (BMD) at various skeletal sites according to serum ferritin quartiles after adjustment for confounders in women ≥45 years of age. Values are presented as the mean with 95 % confidence intervals (CIs). Multivariate-adjusted least-square mean BMD values, in terms of the serum ferritin quartiles, were estimated using analysis of covariance (ANCOVA). Base model: adjustment for age, weight, and height. Multivariable model: adjustment for smoking, drinking, and exercise habits, serum 25-hydroxyvitamin D level, and calcium and phosphorus intake, as well as factors from the base model
Because the International Society for Clinical Densitometry advises that the WHO criteria should not be used in premenopausal women or subject <50 years of age [24], we performed the logistic regression analyses for the prevalent osteoporosis according to serum ferritin quartiles in postmenopausal women and/or women ≥50 years of age (n = 2,712; 81.4 % of women in age group III; mean age = 62.6 ± 9.3 years). The overall proportion of these women who met the criteria for osteoporosis was 31.1 %. The prevalence of osteoporosis from Q1 to Q4 was 24.9, 29.5, 32.6, and 38.0 %, respectively. After adjustment for age, weight, and height, the ORs for prevalent osteoporosis linearly increased across increasing ferritin quartiles, and the odds for prevalent osteoporosis was 47 % higher in women in Q4, compared with those in Q1 (Fig. 2). After adopting the multivariable adjustment model, the ORs in Q3 and Q4 remained statistically significant.
Odds ratios (ORs) and 95 % confidence intervals (CIs) for osteoporosis according to serum ferritin quartiles after adjustment for confounders in postmenopausal women and/or women ≥50 years of age. Osteoporosis was diagnosed by a T-score ≤ −2.5 SD at any of the sites on the lumbar spine, femur neck, or total femur. Base model: adjustment for age, weight, and height. Multivariable model: adjustment for smoking, drinking, and exercise habits, serum 25-hydroxyvitamin D level, and calcium and phosphorus intake, as well as factors from the base model
The overall proportion of women in age group III who had prevalent fractures was 13.3 % (n = 443). In detail, 77 subjects (2.3 %) had osteoporotic fracture history based on a standard self-administered questionnaire (e.g., 16 vertebra, 4 hip, 46 wrist, 3 humerus, 2 clavicle, and 6 rib fractures), and 376 subjects (11.3 %) were considered having suffered from a vertebral fracture by historical height loss of 4.0 cm or more. Ten women met both criteria. The prevalence of fractures from Q1 to Q4 was 8.4, 12.3, 14.7, and 18.7 %, respectively. After considering potential confounders, the ORs for prevalent fractures linearly increased across increasing ferritin quartiles, and the odds for prevalent fractures was 52 % higher in women in Q4, compared with those in Q1 (Fig. 3).
Odds ratios (ORs) and 95 % confidence intervals (CIs) for prevalent fractures according to serum ferritin quartiles after adjustment for confounders in women ≥45 years of age. Prevalent fractures are identified based on self-reported data and a height loss threshold of 4.0 cm, as described in the “Materials and methods.” Base model: adjustment for age, weight, and height. Multivariable model: adjustment for smoking, drinking, and exercise habits, serum 25-hydroxyvitamin D level, and calcium and phosphorus intake, as well as factors from the base model
Among women classified as in age group III, multiple regression analyses revealed that serum ferritin concentrations were positively associated with serum total ALP levels after adjustment for multivariable confounding factors (β = 0.046, SE = 0.009, P < 0.001, R 2 = 0.155). Because increased body iron stores could lead to increased liver iron concentration that might affect serum total ALP levels, we additionally adjusted for liver enzymes, including AST and ALT, and observed that the statistical significance persisted (β = 0.031, SE = 0.009, P = 0.001, R 2 = 0.191).
Discussion
In this large population-based cohort of 14,017 Koreans, we found that serum ferritin concentrations were inversely associated with the BMD values at the lumbar spine, femur neck, and total femur in women ≥45 years of age, classified as age group III, after performing multiple regression analyses. When we divided these women into serum ferritin quartiles, the odds for prevalent osteoporosis and fractures were 1.55- and 1.52-fold higher, respectively, in subjects in the highest quartile compared with those in the lowest quartile after adjustment for potential confounders. This is the first report showing that the significant associations between serum ferritin level and osteoporosis-related phenotypes are observed in a nationally representative cohort as well.
Ferritin is an iron storage molecule that has the capacity to bind up to 4,500 atoms of iron and is known to modulate the potential toxicity of iron [25]. Because this protein can accurately reflect differences in body iron storages by age and sex [26], it has been widely used as a marker of iron status in epidemiological studies [27]. Considered along with a plausible explanation regarding the effects of iron on bone metabolism in experimental and animal data, the consistent results in the present population-based and previous longitudinal studies [11] support the possibility that serum ferritin could be one of useful biomarkers for predicting poor bone health.
The patterns of age-related BMD changes that occur in life vary with differences in ethnic, gender, hormonal, and environmental variables. To investigate the pattern in Koreans, we presented the BMD values at the lumbar spine, femur neck, and total femur by 5 years interval in this cohort as Supplementary Fig. 1. Specifically, women and men reached peak bone mass in the early 20s, although there were some differences in the exact timing depending on the skeletal sites. In women, after a relatively small change in BMD values, BMD dramatically decreased after the mid-40s. The previous study reported that the mean age of natural menopause in Korean women was 47 years, markedly younger than in Caucasians [28, 29]. In addition, a number of studies have observed accelerated bone loss even in perimenopausal women prior to their menopause [30, 31]. Based on these backgrounds and the observed patterns of age-related BMD changes at various skeletal sites in the KNHANES, we adopted 24 and 45 years old as the cutoff points for age stratification in women. In men, age-related bone loss begins immediately after peak bone mass and BMD gradually decreases without a prominent acceleration phase. However, we applied the same criterion with women for grouping to obtain a comparable analysis regarding the association between serum iron-related markers and BMD in the present study.
In agreement with previous studies [32], our study shows that mean serum ferritin levels were markedly lower in women than in men. However, in spite of the lower ferritin levels, a particularly interesting point is that the association between serum ferritin and BMD was only consistently significant in women ≥45 years of age. We speculate that the inconsistent results may be mainly explained by abrupt loss of estrogen in these women, besides the gender difference. In more detail, substantial evidence indicates that estrogen deficiency during menopause can cause relative increases in osteoclastic bone resorption through directly affecting bone cells [33] and indirectly involving diverse mechanisms, such as reactive oxygen species, cytokines, and growth factors [34, 35], resulting in more accelerated bone loss. Therefore, our present results indicate that this dynamic period causing enormous changes in bone metabolism may be the most vulnerable to iron overload and the subsequent increase of oxidative stress. Studies focusing on how male and female hormones interact with iron in relation to bone metabolism may help to explain the differences depending on the age groups and the genders in the association between iron storages and low bone mass. Meanwhile, the association of higher serum ferritin levels with lower BMD in women ≥45 years of age appears to be more prominent at the lumbar spine, which is mainly composed of trabecular bone, than at the proximal femur, which is usually composed of cortical architecture. Because the trabecular bone is known to be more metabolically active after menopause, these results further support the notion that the deleterious effects of iron on bone could be augmented in a state of high bone turnover.
The ultimate goal of bone biology research is to reduce the risk of osteoporotic fractures. Because data from radiographs were not available for this national cohort, as an alternative line of investigation, we assessed the effects on fractures based on self-reported data and a height loss threshold of 4.0 cm as an indicator of prevalent vertebral fractures. Although the self-reported fractures could have been affected by recall bias, they have been demonstrated to be accurate with a specificity above 80 % [36–38] and are rarely underreported [37]. Furthermore, a height loss threshold of 4.0 cm has also been shown to demonstrate a specificity of 98.3 % and a positive predictive value of 63.9 % for the development of new vertebral fractures [22]. In the present study, we identified the women ≥45 years of age in the highest ferritin quartile had significantly higher odds for prevalent fractures, which in consistent with the findings observed in our previous longitudinal study on postmenopausal women [11]. These results validate that there is the presence of gender difference in terms of the effects of iron on fractures as well.
Serum total ALP, normally contributed to by bone and liver isoforms in approximately equal amounts [39], is regarded as a useful marker which can reflect the degree of bone turnover in subjects without liver diseases [40]. Based on this background, we performed the multiple regression analysis and found that the serum ferritin concentrations were positively correlated with the serum total ALP levels in women ≥45 years of age after adjusting for confounders. This result indirectly suggests that the higher prevalence of osteoporosis and fractures observed in women with higher serum ferritin levels may have resulted from the increased bone turnover rate in these subjects.
The major strength of this study is that we analyzed data collected from a nationwide survey of Korea that included more than 14,000 participants of both genders between 10 and 80 years of age. Despite this strength, there are some limitations to this study. Most importantly, the serum ferritin concentration can be elevated in response to systemic inflammation, which is thought to be involved in the pathophysiological mechanisms underlying osteopenia and/or osteoporosis [41]. However, we could not adjust for serum high sensitivity C-reactive protein, a well-known acute-phase reactant [42]. Therefore, there is the possibility that the present results could be biased by this potential confounder. Second, because this is a cross-sectional study, we cannot determine if a causal relationship exists between variables. Furthermore, because serum estrogen level was not measured in the KNHANES, we could not adjust for the menopausal status and the resultant change of this sex hormone in the data analyses. Therefore, some researchers can argue that high serum ferritin level may be just an inactive bystander reflecting low serum estrogen level, based on the recent study reporting the inverse relationship between serum levels of estrogen and ferritin during menopausal transition [43]. We cannot answer this concern from the present study. However, in our longitudinal cohort in which serum estrogen levels were available [11], additional adjustment for estrogen did not attenuate the significant correlation between higher serum ferritin levels and accelerated bone loss at all measured skeletal sites (Supplementary Table 1). Accordingly, we believe that our present results support in vitro and in vivo studies showing the direct deleterious effects of iron on bone metabolism. Third, prevalent fractures were assessed using self-reported data and historical height loss; however, they were not validated by radiographs, and thus, the analyses involving fractures could be seriously biased by the memory of subjects. Fourth, smoking and drinking habits were categorized into three and two levels, respectively, and thus, this simple classification may not fully reflect the dose dependent effects of these variables on bone health. Finally, although we attempted to consider as many confounding factors as possible, we cannot exclude the possibility that the observed association could be attributable to uncontrolled factors that affect serum ferritin levels and/or BMD values.
In summary, data gathered from a nationally representative cohort demonstrate that higher body iron stores are significantly associated with lower bone mass at various skeletal sites and the increased prevalence of osteoporosis and fractures, especially in women ≥45 years of age. The present study may have clinical implications in that it provides the first evidence that the associations between serum ferritin level and bone parameters are the most prominent in women ≥45 years of age and suggests that the measurement of serum ferritin levels may provide additional information for predicting poor bone health outcomes in these women. Further interventional studies are needed to confirm the causal role of body iron stores in humans.
References
McCord JM (1996) Effects of positive iron status at a cellular level. Nutr Rev 54:85–88
Andrews NC (1999) Disorders of iron metabolism. N Engl J Med 341:1986–1995
Wilson JG, Lindquist JH, Grambow SC, Crook ED, Maher JF (2003) Potential role of increased iron stores in diabetes. Am J Med Sci 325:332–339
Ishii KA, Fumoto T, Iwai K et al (2009) Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med 15:259–266
Yamasaki K, Hagiwara H (2009) Excess iron inhibits osteoblast metabolism. Toxicol Lett 191:211–215
Yang Q, Jian J, Abramson SB, Huang X (2011) Inhibitory effects of iron on bone morphogenetic protein 2-induced osteoblastogenesis. J Bone Miner Res 26:1188–1196
Tsay J, Yang Z, Ross FP et al (2010) Bone loss caused by iron overload in a murine model: importance of oxidative stress. Blood 116:2582–2589
Vogiatzi MG, Macklin EA, Fung EB et al (2009) Bone disease in thalassemia: a frequent and still unresolved problem. J Bone Miner Res 24:543–557
Guggenbuhl P, Deugnier Y, Boisdet JF, Rolland Y, Perdriger A, Pawlotsky Y, Chales G (2005) Bone mineral density in men with genetic hemochromatosis and HFE gene mutation. Osteoporos Int 16:1809–1814
Sinigaglia L, Fargion S, Fracanzani AL, Binelli L, Battafarano N, Varenna M, Piperno A, Fiorelli G (1997) Bone and joint involvement in genetic hemochromatosis: role of cirrhosis and iron overload. J Rheumatol 24:1809–1813
Kim B-J, Ahn SH, Bae SJ, Kim EH, Lee S-H, Kim H-K, Choe JW, Koh J-M, Kim GS (2012) Iron overload accelerates bone loss in healthy postmenopausal women and middle-aged men: a 3-year retrospective longitudinal study. J Bone Miner Res 27:2279–2290
Kular J, Tickner J, Chim SM, Xu J (2012) An overview of the regulation of bone remodelling at the cellular level. Clin Biochem 45:863–873
The Division of Chronic Disease Surveillance, Korea Centers for Disease Control and Prevention (2008–2010) The Fourth and Fifth Korea National Health and Nutrition Examination Survey (KNHANES IV & V)
Halle M, Konig D, Berg A, Keul J, Baumstark MW (1997) Relationship of serum ferritin concentrations with metabolic cardiovascular risk factors in men without evidence for coronary artery disease. Atherosclerosis 128:235–240
Lee BK, Kim Y, Kim YI (2011) Association of serum ferritin with metabolic syndrome and diabetes mellitus in the South Korean general population according to the Korean National Health and Nutrition Examination Survey 2008. Metabolism 60:1416–1424
Schoeller DA, Tylavsky FA, Baer DJ et al (2005) QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr 81:1018–1025
Kelly TL, Wilson KE, Heymsfield SB (2009) Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One 4:e7038
Lee EY, Kim D, Kim KM, Kim KJ, Choi HS, Rhee Y, Lim SK (2012) Age-related bone mineral density patterns in Koreans (KNHANES IV). J Clin Endocrinol Metab 97:3310–3318
Myong JP, Kim HR, Choi SE, Koo JW (2012) Dose-related effect of urinary cotinine levels on bone mineral density among Korean females. Osteoporos Int. doi:10.1007/s00198-012-2107-6
Ettinger B, Black DM, Nevitt MC, Rundle AC, Cauley JA, Cummings SR, Genant HK (1992) Contribution of vertebral deformities to chronic back pain and disability. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res 7:449–456
Kleerekoper M, Nelson DA, Peterson EL, Tilley BC (1992) Outcome variables in osteoporosis trials. Bone 13(Suppl 1):S29–S34
Siminoski K, Jiang G, Adachi JD et al (2005) Accuracy of height loss during prospective monitoring for detection of incident vertebral fractures. Osteoporos Int 16:403–410
Drake MT, Khosla S (2012) Male osteoporosis. Endocrinol Metab Clin North Am 41:629–641
Baim S, Binkley N, Bilezikian JP, Kendler DL, Hans DB, Lewiecki EM, Silverman S (2008) Official Positions of the International Society for Clinical Densitometry and Executive Summary of the 2007 ISCD Position Development Conference. J Clin Densitom 11:75–91
Harrison PM, Arosio P (1996) The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta 1275:161–203
Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM (2000) Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J 140:98–104
Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB (2004) Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA 291:711–717
Hong JS, Yi SW, Kang HC, Jee SH, Kang HG, Bayasgalan G, Ohrr H (2007) Age at menopause and cause-specific mortality in South Korean women: Kangwha Cohort Study. Maturitas 56:411–419
Greendale GA, Lee NP, Arriola ER (1999) The menopause. Lancet 353:571–580
Recker R, Lappe J, Davies K, Heaney R (2000) Characterization of perimenopausal bone loss: a prospective study. J Bone Miner Res 15:1965–1973
Sirola J, Kroger H, Honkanen R, Jurvelin JS, Sandini L, Tuppurainen MT, Saarikoski S (2003) Factors affecting bone loss around menopause in women without HRT: a prospective study. Maturitas 45:159–167
Kim CH, Kim HK, Bae SJ, Park JY, Lee KU (2011) Association of elevated serum ferritin concentration with insulin resistance and impaired glucose metabolism in Korean men and women. Metabolism 60:414–420
Feng X, McDonald JM (2011) Disorders of bone remodeling. Annu Rev Pathol 6:121–145
Raisz LG (2005) Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 115:3318–3325
Weitzmann MN, Pacifici R (2006) Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest 116:1186–1194
Hundrup YA, Høidrup S, Obel EB, Rasmussen NK (2004) The validity of self-reported fractures among Danish female nurses: comparison with fractures registered in the Danish National Hospital Register. Scand J Public Health 32:136–143
Ivers RQ, Cumming RG, Mitchell P, Peduto AJ (2002) The accuracy of self-reported fractures in older people. J Clin Epidemiol 55:452–457
Nevitt MC, Cummings SR, Browner WS, Seeley DG, Cauley JA, Vogt TM, Black DM (1992) The accuracy of self-report of fractures in elderly women: evidence from a prospective study. Am J Epidemiol 135:490–499
Green S, Anstiss CL, Fishman WH (1971) Automated differential isoenzyme analysis. II. The fractionation of serum alkaline phosphatases into "liver", "intestinal" and "other" components. Enzymologia 41:9–26
van Straalen JP, Sanders E, Prummel MF, Sanders GT (1991) Bone-alkaline phosphatase as indicator of bone formation. Clin Chim Acta 201:27–33
Koh JM, Khang YH, Jung CH, Bae S, Kim DJ, Chung YE, Kim GS (2005) Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. Osteoporos Int 16:1263–1271
Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340:448–454
Jian J, Pelle E, Huang X (2009) Iron and menopause: does increased iron affect the health of postmenopausal women? Antioxid Redox Signal 11:2939–2943
Acknowledgments
This study was supported by a grant from the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (project number A110536).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(JPEG 12 kb)
Rights and permissions
About this article
Cite this article
Kim, BJ., Lee, S.H., Koh, JM. et al. The association between higher serum ferritin level and lower bone mineral density is prominent in women ≥45 years of age (KNHANES 2008–2010). Osteoporos Int 24, 2627–2637 (2013). https://doi.org/10.1007/s00198-013-2363-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-013-2363-0