Abstract
Although trials have shown that exercise has positive effects on bone mineral density (BMD), the majority of exercise trials have been conducted in older women. The aim of this study was to systematically review trials examining the effect of weight-bearing and resistance-based exercise modalities on the BMD of hip and lumbar spine of middle-aged and older men. Eight electronic databases were searched in August 2012. Randomised controlled or controlled trials that assessed the effect of weight-bearing and resistance-based exercise interventions on BMD measured by dual-energy x-ray absorptiometry, and reported effects in middle-aged and older men were included. Eight trials detailed in nine papers were included. The interventions included walking (n = 2), resistance training (n = 3), walking + resistance training (n = 1), resistance training + impact-loading activities (n = 1) and resistance training + Tai Chi (n = 1). Five of the eight trials achieved a score of less than 50 % on the modified Delphi quality rating scale. Further, there was heterogeneity in the type, intensity, frequency and duration of the exercise regimens. Effects of exercise varied greatly among studies, with six interventions having a positive effect on BMD and two interventions having no significant effect. It appears that resistance training alone or in combination with impact-loading activities are most osteogenic for this population, whereas the walking trials had limited effect on BMD. Therefore, regular resistance training and impact-loading activities should be considered as a strategy to prevent osteoporosis in middle-aged and older men. High quality randomised controlled trials are needed to establish the optimal exercise prescription.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the ageing of the population, developing safe and effective strategies to prevent osteoporosis and consequent fractures is of great importance. The mechanisms that underpin bone mineral density (BMD) decline following peak bone mass are multifaceted and complex in nature. Although changes in sex hormones, nutrition and bone-loading are responsible for bone loss across the lifespan in males and females, important gender-specific differences exist [1, 2]. The decline in bone mass in men up to the age of 50 and in premenopausal women is approximately 0.3 to 1.1 % per year [3], with an accelerated rate of bone loss in women for 4 to 8 years following menopause [4] due to oestrogen withdrawal. During this period, women will lose approximately 15 % in BMD or one standard deviation, leading to a 1.5-to 3-fold increase in fracture risk [5, 6]. In contrast, the decline in bone mass for men is more gradual with an age-related loss of ~0.7 % per year after the age of 50 [3]. Nonetheless, approximately one third of all osteoporotic fractures are accounted for by middle-aged and older men [7], and so understanding the role preventative strategies, (for example exercise) may have in attenuating the bone loss experienced by men in this age group is of great importance.
Regular physical exercise has been recommended as a low-cost and safe non-pharmacological strategy to counter the loss of bone mass that accompanies ageing. The principles of effective bone loading are somewhat unique compared to the exercise response of other body systems such as the muscular or cardiovascular systems. It has long been established that to improve bone density, bone tissue must be subjected to mechanical loading above that experienced in daily activities [8]. Mechanical loading should be dynamic, novel and involve high strain magnitudes and rates resulting in substantial overload [8, 9].
To date, the effects of exercise on the skeleton have been examined predominantly in pre- and post-menopausal women [10–17] due to the higher rates of osteoporosis in women than in men. Reviews of these exercise trials indicate that, in women, the combination of high-impact loading exercises and moderate to high intensity resistance training is the most beneficial to prevent age-related bone loss [13–15, 17, 18]. However, older women not only have different rates of bone loss compared to older men, but during menopause the skeleton's response to loading is dampened [19] due to the reduced sensitivity of bone cells [20]. Consequently, the response of bone to exercise is dissimilar between middle-aged men and women during the first few years following the onset of menopause [21].
As the burden of osteoporosis in men is becoming increasingly recognised [22], a small but growing number of exercise interventions have been trialled in men. Recent reviews of the effect of exercise on the BMD of male and female adults [23] and older adults [18] have focused on the effects on BMD in women. To our knowledge, only one review by Kelley and colleagues [24] has exclusively focused on the effect of exercise interventions on BMD in men. Of the eight interventions included in the review [24], only two used dual x-ray absorptiometry (DXA) to measure BMD of middle-aged or older male participants, while the remaining studies recruited exclusively younger participants or used other methods to assess BMD. The authors [24] concluded that exercise may help improve or maintain bone density, but that more trials were required to confirm the benefits in men. However, the review by Kelley et al. [24] was published over a decade ago and therefore an updated review of the effects of exercise on the bone health of middle-aged and older men is warranted.
Moreover, there is some discrepancy between the conclusions of bone and exercise reviews [17, 18, 23] and recent international practise guidelines for osteoporosis in men [22]. These new guidelines recommend activities such as walking as a preventative strategy for osteoporosis despite an apparent lack of supporting evidence from randomised controlled trials. Whilst walking is beneficial for an array of health outcomes, its prescription as a stand-alone osteoporosis prevention strategy is inconsistent with the current American College of Sports Medicine (ACSM) position stand on physical activity for bone health [25].
With an ageing population and thus an increasing prevalence of osteoporosis in men, there is a growing urgency for health professionals to develop evidence-based exercise guidelines for men. Therefore, the aim of this systematic review was to examine both the findings and the study quality of exercise trials examining the effect of weight-bearing and resistance-based modalities on BMD of the hip and lumbar spine in middle-aged and older men.
Methods
Literature search
We conducted searches in the databases PubMed, EMBASE (via EMBASE.com), CENTRAL (Cochrane Central Register of Controlled Trials), PEDro and SPORTDiscus in August 2012. Search terms included combinations of thesaurus terms (MeSH in PubMed, CENTRAL, EMtree in EMBASE) and free text terms. Free terms for exercise (‘resistance*training’, ‘strengthening’, ‘exercise’, ‘weight*lifting’, ‘weight*bearing’, ‘jump*’, ‘bounding’, ‘skipping’, ‘hopping’, ‘impact*loading’, ‘high*impact’, ‘running’, ‘stair*climbing’, ‘jogging’, ‘walk*’, ‘leap*’, ‘weight*training’, ‘resistance’, ‘strength’) were used in AND-combination with the search terms expressing the target population (‘men’, ‘adults’, ‘patients’, ‘participants’, ‘subjects’, ‘people’, middle*aged’, ‘aged’, ‘aged, 80 and over’) and search terms representing bone density (e.g. ‘bone*density’, ‘bone*strength’, ‘bone*mass’, ‘bone*mineral’, ‘bone*tissue’, ‘metabolic*bone*disease’). In PubMed, search results were limited by search terms indicating specific study designs (e.g. ‘trial’, ‘random’, ‘intervention’, ‘pilot*study’). The complete list of search terms is available on request.
Inclusion criteria
The inclusion criteria were: (1) design: randomised controlled trials (RCT) or controlled trials (CT); (2) population: middle-aged or older men (45 years and older). Studies in middle-aged and older men and studies including men and women in which results for men and women were reported separately were eligible for inclusion; (3) intervention: any exercise protocol involving resistance training only, impact loading exercise only, weight-bearing aerobic exercise only or a combination of these types of exercise; and (4) outcome: BMD (g/cm2) of the lumbar spine, Ward's triangle, trochanter, proximal femur, femoral neck or total hip measured by DXA. Only full-text articles were included, and no restrictions were placed on the language of the article. Titles and abstracts of articles identified through the search process were reviewed first by K.A.B to exclude articles out of scope. Subsequently, K.A.B., J.G.Z.v.U. and D.R.T. independently reviewed the full texts of all potentially relevant articles for eligibility. Disagreements were discussed and resolved. Articles that met the inclusion criteria were also examined to ensure that the same subjects were not included in more than one article based on data from the same study. Reference lists of eligible articles were manually checked for additional references.
Quality assessment and data extraction
Data on the study population, exercise programmes and outcome measures were extracted independently by two authors (K.A.B. and D.R.T.). On the basis of programme descriptions in the individual studies, programmes were qualified by an exercise physiologist as weight-bearing aerobic, strength training, impact-loading exercise or a combination thereof. Methodological quality of the included reviews was independently determined by two of the three authors (K.A.B. and D.R.T. or J.G.Z.v.U.) using the Delphi list developed by Verhagen et al. [26]. This list consists of nine quality criteria assessing different methodological aspects. Two of the nine criteria (i.e. blinding of the trainers and blinding of the participants) were not appropriate for the type of interventions we were reviewing, and these items were excluded. Thus, quality of included studies was examined using a seven item quality rating list [26, 27].
-
1.
Was the method of randomisation performed?
-
2.
Was the treatment allocation concealed?
-
3a.
Were the groups similar at baseline regarding the most prognostic indicators?
-
3b.
If groups weren't similar at baseline, was this adjusted for in the analyses?
-
4.
Were the eligibility criteria specified?
-
5.
Was the outcome assessor blinded?
-
6.
Were point estimates and measures of variability presented for BMD?
-
7.
Did the analysis include an intention-to-treat analysis (ITT) (defined as all of participants randomised were included in analysis)?
All criteria were equally rated using a ‘yes’ (1), ‘no’ (0), or ‘unclear’ (0) answer format, and a quality score was generated as a percentage of the maximum score for each included study.
Results
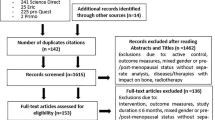
The systematic search resulted in 3,106 records; details of the search process are shown in Fig. 1. Abstracts of 3,106 articles were initially reviewed. After removing articles out of scope, the full text of 42 articles was independently checked for eligibility. Thirty-three articles were excluded. Checking the reference lists of eligible articles did not result in additional articles. Nine articles from eight studies met the inclusion criteria [28–36]. One intervention was described in two articles, but with different durations of intervention [30, 31]. Both articles are included and are considered as the one intervention.
Quality assessment
The results of the methodological quality assessment are presented in Table 1. Quality scores ranged from 29 to 100 % with three of the eight studies scoring over 50 %. Although six of the included studies were RCTs, randomisation was not concealed in two of these RCTs. Methodological aspects that were not scored positively in most of the eight included studies were reporting of point estimates (included in one study [30, 31]), blinding of the outcome assessor (included in three studies [28, 30, 31, 33]) and conducting an ITT analysis (included four studies [30–33, 36]). All of the studies scored well for group similarity at baseline [28–36] and five of the eight specified eligibility criteria [28–32, 35].
Study population and exercise programmes
Characteristics of the study participants and exercise programmes are shown in Table 2. Participants in the studies were middle-aged and older men predominantly from non-clinical populations, but one study included heart transplant patients on glucocorticoid treatment [36]. Sample sizes ranged from 11 to 147 men, with participants aged 50 to 79 years. The duration of the programmes ranged from 3 months to 4 years (mean 13 months) with DXA assessments at the start and after completion of the exercise programmes. In the heart transplantation study, Braith and colleagues [36] also measured the BMD of participants 2 months prior to commencing the exercise intervention to assess the impact of glucocorticoid therapy following transplantation. Changes in BMD over the initial 2 months prior to exercise are also reported in Table 2. Of the eight exercise programmes, two included walking only, three included resistance training only, one included walking and resistance training, one included resistance training and impact-loading activities and one included resistance training and Tai Chi. The majority of the programmes prescribed three exercise sessions a week (ranging from 2–5 each week). The prescribed intensities of the interventions varied greatly. Within the trials that included resistance exercises, intensity in all but one of the interventions used individualised intensities; percentage of 1-RM [30, 31], where RM or repetition maximum is the maximum amount that can be moved or lifted one time only, 8-RM [29] or 5–15-RM [35]. The exception was the study by Woo et al. [28], who did not report intensity but instead the participants were supplied with a medium strength elastic band (Theraband) for resistance. The two trials involving aerobic exercise required the participants to walk at a brisk pace corresponding to 40–60 % of their maximal oxygen uptake [34] or their lactate threshold [33]. The jumping programme in the trial by Kukuljan and colleges [30, 31] required the participants to perform impact-loading activities with ground reaction forces ranging from 1.5 to 9.7 times body weight.
Four of the eight trials were supervised, and in the majority of cases this was by exercise specialists/exercise physiologists. In addition to an exercise specialist, in the trial by Menkes and colleagues [35], registered nurses and physical therapists were the supervisors. Three of the studies did not report if the sessions were supervised [28, 32, 33], and one was unsupervised [34]. Machine and free weights were used in all of the resistance training protocols with the exception of the one trial that used elastic bands [28]. No equipment was used by the participants in the walking interventions, while the impact-loading intervention used boxes and benches [30, 31]. While all of the eight studies included control groups, only four described the instructions given to these participants, and these were poorly detailed [28, 29, 34, 36]. Braith et al. [36] compared their intervention with a post-operative walking programme; Huuskonen et al. [34] advised the control group participants to make their personal choice whether to engage in physical activity or not; Whiteford and colleagues [29] provided their control group with education and advised them to walk for 30 min 3 days per week, and Woo et al. [28] reported that the control group was not prescribed any exercise.
Reported findings
The effects of the interventions on BMD are shown in Table 2. BMD of the lumbar spine was reported in seven of the eight studies [28, 29, 31, 32, 34–36]. In addition, femoral neck BMD was reported in five [29, 31, 32, 35, 36], total hip BMD in three [28, 29, 31], trochanteric BMD in three [29, 31, 32], both Ward's triangle [32] and proximal femur BMD in one [34], while Paillard and colleagues [33] reported only that they measured hip BMD.
The greatest between group change in BMD was in the trial by Braith et al. [36] among heart transplant patients. The only study to include high-impact loading exercise or high-velocity power resistance training in their trial was Kukuljan et al. [31]. Although there were significant increases in BMD in most of the resistance training programmes, the study by Kukuljan et al. was the only trial to report a significant difference in BMD (femoral neck) between the exercise and control group following the intervention period. Both the exercise and control group lost BMD in the trials by Paillard et al. [33], Ryan et al. [32] and Whiteford et al. [29] with the exception of the increase in femoral neck BMD of the exercising group in the study by Ryan et al. [32]. Importantly, although not statistically significant, the exercise groups all lost less BMD than the control groups, in all but one of the studies [28]. Conversely, in the trial by Woo et al. [28], total hip BMD of those in the elastic band resistance group declined more than individuals in the control group, although lumbar spine BMD of the control and exercise groups increased. Between group differences for two of the studies [34, 35] could not be calculated because numerical data were not reported in these papers.
Dropout, attendance and adverse events
Four of the eight studies reported no dropouts from the control or exercise groups [32, 33, 35, 36]. Of the trials that did report dropout, the average rate was 3.3 % [28, 29, 31, 34]. For the four studies [28–31, 35] who reported dropout by group, the overall dropout rates were 6.8 and 2.1 % for the exercise and control groups, respectively. Reason for drop out included personal reasons [29, 34], death of participants [34], illness [29, 31] or work and personal commitments [29, 31]. Only four of the eight studies reported attendance rates [28–31, 35]. Six of the eight studies did not include or report any adverse events [28, 32–36]. In the two studies that did, Kukuljan and colleagues [30, 31] noted that although there were no serious or adverse events associated with their exercise regimen (exercise only and the exercise and milk groups), a number of medical complaints occurred. These included exacerbation of longstanding gout of the foot (n = 1), aggravated knee or hip pain (n = 2), lower back injury (n = 2) and aggravation of a long standing shoulder injury (n = 2). In addition, three men suffered an inguinal hernia. All of these men were able to continue with the programme except for one man whose longstanding lower back injury caused him to withdraw from the trial. Similarly, Whiteford et al. [29] noted the following as reasons given for withdrawal from their resistance training programme: bypass surgery (n = 1), fracture of a thoracic vertebra (n = 1), hip replacement (n = 1), depression (n = 1), hip problems (n = 1), chronic illness (n = 1), moved (n = 3), and personal reasons (n = 7). These reasons were not reported as adverse events associated with or as a result of participation in the resistance training programme. Five men in the trial by Whiteford et al. [29] withdrew from the control group due to depression (n = 1), moved away from the study location (n = 3) or for personal reasons (n = 1).
Discussion
The purpose of this systematic review was to investigate the effects of exercise on hip or spine BMD in middle-aged and older men. Following a search of the literature, we identified eight intervention studies reported in nine journal articles that met the inclusion criteria. The results from this review support the findings of similar reviews in pre- and post-menopausal women that resistance training and high-impact loading activities are more likely to induce positive effects on the BMD of weight-bearing skeletal sites than walking, which is relatively low-impact. Nevertheless, the optimal exercise prescription for middle-aged and older men cannot be determined from the results of the trials in this systematic review due to variations in reported BMD changes and in the design of the exercise programmes and the relatively poor methodological quality of a number of the trials.
Five of the eight trials achieved a quality rating score of less than 50 % on the modified Delphi rating scale, and therefore caution should be taken when interpreting the results of these studies due to their methodological quality. Further, essential information regarding methodological quality was missing in all included studies, even if the trial itself was methodologically sound, and for all but one of the studies it was necessary to request further information from the authors.
Of the studies scoring higher on the Delphi quality rating scale, only Kukuljan and colleagues [30, 31] scored positively on all methodological items. Their trial of resistance training and high-impact loading exercise was also the only trial to find significant between group effects at the femoral neck favouring the exercise group. However, this significant difference between the exercise and control group was not mirrored at the other measured sites (lumbar spine or total hip). Woo et al. [28] who scored the second highest quality rating reported that there was no significant differences between the groups at either the spine or hip site although only elastic bands were used as the resistance. Whiteford et al. [29] who scored 57 % also found no significant differences in BMD between the resistance training and the control groups following the intervention. All but one of the trials [34] that scored less than 50 % reported that exercise had a positive effect on BMD. It is important to note that two of the four exercise interventions that reported significant within group improvements in BMD allowed participants to choose their group allocation. This non-random allocation may have been a further confounding factor within these studies, and results from these two trials should be interpreted cautiously. Methodological aspects that should be improved in future studies include providing point estimates and measures of variability, blinding the outcome assessor, concealing the treatment allocation and including an intention-to-treat analysis. It is important to describe this well in future studies so that readers can appropriately appraise the quality of the study.
In addition to methodological quality and reporting, the appropriateness of the design of exercise trials must be considered when drawing conclusions from the results. Only four of the eight studies [28–31, 35] recorded and reported attendance rates of the exercise groups. Further, none of the eight studies reported adherence, which should not be confused with attendance. A participant can attend an exercise session but might not adhere to the exercises as prescribed in terms of intensity, etc. The fact that this was not consistently reported in the studies included in this review is an important limitation, which could have resulted in an underestimation of the true effect of exercise on BMD if attendance and adherence rates were low. Consequently, we strongly suggest that both attendance and adherence rates be reported in future intervention studies examining the effect of exercise on BMD. In addition, improvements in bone density are relatively modest with exercise and occur over a prolonged period of time due to the length of the remodeling cycle [25]. A recent review of exercise regimens [18] showed that exercise regimens that were effective in improving the BMD of women were commonly 12 months or longer in duration. In the current review, only four of the eight trials [28–31, 34] were 12 months or more in duration, and therefore the results from trials of shorter duration may not accurately reflect the effect these exercise modalities may have on BMD and must be interpreted with caution.
It is also well established that the intensity and novelty of the load are two of the most important training characteristics that influence the effect of exercise on bone [37]. Bone adapts to habitual loads and without progressing the intensity of the mechanical loads with exercise, BMD will likely be maintained rather than improved [38]. Despite this, the intensity of the exercise programmes was not progressed in three of the eight trials [28, 33, 34]. Furthermore, the description of the rate of progression in the five studies that did include progression was generally lacking in details. Given the importance of progression rather than customary loads to bone adaptation, researchers should aim to make intensity progression a focus when developing new protocols. Less is known about the optimal frequency of exercise for bone health. A recent randomised controlled trial in women by Bailey and colleagues [39] found that brief bouts of impact loading exercise were more beneficial when completed daily than 4 days a week. While both of these frequencies induced increases in bone density, those in the group that exercised 2 days a week saw no change in their BMD and the control group (no exercise) lost BMD. Five of the eight protocols in this systematic review required participants to exercise 3 days each week. Therefore, it may be the case that the frequencies of these studies were not optimal for improving bone health. However, compliance to exercise sessions is an important public health issue and in light of the already low levels of physical activity participation amongst the population, the challenges associated with asking individuals to exercise daily are significant. Trials that further our understanding of the dose–response relationship between exercise and bone are certainly required, particularly in men.
In comparison to the considerably larger number of exercise studies in middle-aged and older women, the existing comparable studies in men are generally shorter in duration and have fewer participants. Furthermore, a greater number of impact-loading exercise interventions have been conducted in women than in men. Although the results from the trials examined in this systematic review are similar to the existing literature in pre- and post-menopausal women [18, 40, 41], further trials in middle-aged and older men are warranted.
Only five of the eight studies included the participants' level of exercise participation in their exclusion criteria. Ryan et al. [32] and Menkes et al. [35] stated that regular exercise participation was part of their exclusion criteria, but did not define the levels of participation that were acceptable. Woo et al. [28] stated that eligible subjects could not be participating in Tai Chi or resistance training at the time of entering the study, which does not capture past exercise participation. Similarly, Whiteford et al. [29] specified that the participants should not be participating in ‘brisk walking’; however, like Woo et al. [28], their inclusion criteria did not capture the participants' level of exercise on entry into the study nor did it exclude regular walkers exercising at lesser intensities. Given that bone adapts favourably to novel stimuli, failing to assess and control for the participants' current level of exercise participation may explain the different responses to a particular exercise modality. Further, only three of the eight interventions reported the calcium levels of the participants [29–31, 34], and fewer still also reported vitamin D status [30, 31]. Given the important roles of calcium and vitamin D in bone metabolism [42], it would be prudent to suggest that data on calcium and vitamin D levels should be included in future exercise trials. The exclusion criteria developed by Kukuljan et al. [31] were highly specific and thus more clearly described the study population. Future studies should aim to include this level of detail when designing and reporting the details of trials.
Results of recent meta-analyses of different exercise modalities (aerobic, resistance training and impact-loading) on BMD in post-menopausal women [43–45] support the findings of the current review in that the effect of exercise on bone density of older adults appears to be modality and intensity-dependent. Specifically, it appears that resistance training alone or in combination with high-impact loading activities has the potential to attenuate or reverse the decline of BMD in middle-aged and older men. Including resistance training would also result in improved physical function [46] and a reduction in falls risk due to increased muscle strength, therefore reducing the risk for fracture [47]. While men should engage in regular walking due to its positive effect on cardiovascular, metabolic and psychosocial health, the evidence from this review does not support the inclusion of walking alone in exercise recommendations for the targeted prevention of osteoporosis in middle-aged and older men. This finding is supported by the results of recent reviews that indicated that walking alone was not effective in increasing the bone density of older women [43, 44]. Nevertheless, a trial in peri-menopausal women indicated that bone density at the femoral neck was maintained following a programme of brisk walking and jogging [45]. Further, brisk walking has been shown to have a positive effect on hip and spine BMD of post-menopausal women [46, 47]. Although it would seem prudent to suggest that men may also benefit from brisk walking, future trials are needed to confirm this recommendation. Consequently, recent guidelines for osteoporosis in men [22] that have recommended walking alone as an osteoporosis prevention strategy are not consistent with the current evidence base on the effect of exercise on BMD in men.
While there is a need to determine the optimal exercise prescription for ‘healthy’ older adults, clinical populations at risk for bone health issues may have the most to gain from undertaking an appropriate osteogenic exercise programme. One of the eight trials in this review was conducted in heart transplant patients, receiving glucocorticoid treatment which has a deleterious effect on bone [36]. The extent to which exercise improved the bone density in this clinical group of patients was most likely due to the rapid rate of treatment-related bone loss immediately prior to the exercise intervention. Hence, these noteworthy changes in BMD are clearly not replicated in healthy men of a comparable age. Despite this, these positive effects are in accordance with results of trials involving clinical populations similarly at risk of accelerated rates of bone loss such as men receiving androgen suppression therapy for prostate cancer [48] and women with breast cancer [49, 50] and thus would support the inclusion of exercise training as an adjuvant therapy for individuals at risk of experiencing treatment-related bone loss. Despite the great potential that exercise may have in managing treatment-related side effects, further trials are needed to determine the optimal exercise prescription for at risk clinical populations.
While resistance training alone or a combination of resistance training and high-impact loading activities appear to be safe and effective in preventing or reversing age-related bone loss in middle-aged and older men, the optimal frequency, session duration, intensity and exact exercise combination cannot be determined from the results of this systematic review. In comparison to the large number of exercise trials in women, osteoporosis prevention exercise trials in older men are sparse. Accordingly, the authors of the ACSM physical activity and bone health position stand [25] have based their recommendations for older adults predominantly from the results of trials in women. We propose that before the optimal exercise prescription to prevent osteoporosis in middle-aged and older men can be prescribed, methodologically robust, long-duration randomised controlled trials in this population are required. Given that gender-specific factors influence bone metabolism, where trials recruit men and women, analysis should be conducted by gender. Trials in this area are logistically challenging due to attrition, adherence and the high costs of undertaking relatively long-duration interventions, however, given the ageing of the population and the proportion of men potentially at risk for osteoporosis, efforts to address the osteogenic exercise requirements for men are urgently required.
This systematic review has several limitations that are worthy of comment. First, our analysis includes only data from published studies and the possibility exists of missing relevant unpublished trials. Second, the relatively small number of participants in a number of these studies may limit the ability to detect a statistically significant difference between the intervention and control groups, and this should be considered when interpreting the results of these studies. As a result, it is strongly suggested that statistical power calculations be included in reports of future intervention studies. Third, many of the studies included in this review did not report the post-intervention scores, or post-intervention data were not available. In addition to this, due to the heterogeneous nature of the included exercise protocols and the variation in BMD measurement sites, a meta-analysis was not performed. Lastly, it must be noted that using BMD as measured by DXA is a further limitation of this review due to concerns regarding the inherent inaccuracies of this method of measurement and its inability to provide information regarding important determinants of bone strength (size, shape and structure) [51]. Consequently, there is growing interest in using quantitative computed tomography (QCT) to assess bone strength, and researchers should aim to use this method to assess the effects of exercise interventions on whole bone strength where possible. To the best of our knowledge, only one exercise trial has used QCT to assess bone strength in middle-aged and older men [31]. While there is a possibility that some studies were missed in the literature search, it is more likely that the small number of trials in this systematic review reflects the strong focus on preventing osteoporosis in women rather than in men.
Safety aspects
Although resistance training and impact-loading activities appear to be safe methods of exercise training, older adults should be carefully screened and supervised prior to and during exercise participation to ensure safety and correct technique. Where appropriate, clinicians should refer patients to appropriate health professionals, such as exercise physiologists or physiotherapists, trained to prescribe exercise for individuals with chronic disease and associated co-morbidities.
Conclusion
Results from this systematic review indicate that resistance training alone or in combination with impact-loading activities is safe and may assist in the prevention of osteoporosis in middle-aged and older men. However, due to the variation among studies as well as in study quality, additional high-quality randomised controlled trials in this population are required in order to establish evidence-based guidelines for the optimal exercise prescription. Nevertheless, for those individuals willing and able to perform physical exercise, regular resistance training and impact-loading activities should be considered as an effective strategy to prevent osteoporosis in middle-aged and older men.
References
Khosla S (2012) Pathogenesis of age-related bone loss in humans. J Gerontol Ser A Biol Sci Med Sci
Amin S, Khosla S (2012) Sex- and age-related differences in bone microarchitecture in men relative to women assessed by high-resolution peripheral quantitative computed tomography. J Osteoporos 2012:129760
Warming L, Hassager C, Christiansen C (2002) Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int 13:105–112
Khosla S, Riggs BL (2005) Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin N Am 34:1015–1030, xi
Kanis JA (2002) Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359:1929–1936
Marcus R (2002) Post-menopausal osteoporosis. Best Pract Res Clin Obstet Gynaecol 16:309–327
Ryan CS, Petkov VI, Adler RA (2011) Osteoporosis in men: the value of laboratory testing. Osteoporos Int 22:1845–1853
Frost HM (1987) Bone “mass” and the “mechanostat”: a proposal. Anat Rec 219:1–9
Rubin CT, Lanyon LE (1984) Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am Vol 66:397–402
Kelley GA (1998) Exercise and regional bone mineral density in postmenopausal women: a meta-analytic review of randomized trials. Am J Phys Med Rehabil 77:76–87
Nikander R, Sievanen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P (2010) Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC medicine 8:47
Wolff I, van Croonenborg JJ, Kemper HC, Kostense PJ, Twisk JW (1999) The effect of exercise training programmes on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int 9:1–12
Berard A, Bravo G, Gauthier P (1997) Meta-analysis of the effectiveness of physical activity for the prevention of bone loss in postmenopausal women. Osteoporos Int 7:331–337
Kelley GA, Kelley KS (2006) Exercise and bone mineral density at the femoral neck in postmenopausal women: a meta-analysis of controlled clinical trials with individual patient data. Am J Obstet Gynecol 194:760–767
Kelley GA, Kelley KS, Tran ZV (2002) Exercise and lumbar spine bone mineral density in postmenopausal women: a meta-analysis of individual patient data. In The journals of gerontology Series A, Biological sciences and medical sciences M599–604
Borer KT (2005) Physical activity in the prevention and amelioration of osteoporosis in women: interaction of mechanical, hormonal and dietary factors. Sports Med 35:779–830
Schmitt NM, Schmitt J, Doren M (2009) The role of physical activity in the prevention of osteoporosis in postmenopausal women-An update. Maturitas 63:34–38
Gómez-Cabello A, Ara I, González-Agüero A, Casajüs JA, Vicente-Rodríguez G (2012) Effects of training on bone mass in older adults: a systematic review. Sports Med 42:301–325
Lanyon LE (1996) Using functional loading to influence bone mass and architecture: objectives, mechanisms, and relationship with oestrogen of the mechanically adaptive process in bone. Bone 18:37S–43S
Lee KC, Lanyon LE (2004) Mechanical loading influences bone mass through oestrogen receptor alpha. Exerc Sport Sci Rev 32:64–68
Bassey EJ, Rothwell MC, Littlewood JJ, Pye DW (1998) Pre- and postmenopausal women have different bone mineral density responses to the same high-impact exercise. J Bone Miner Res 13(12):1805–1813
Watts NB, Adler RA, Bilezikian JP, Drake MT, Eastell R, Orwoll ES, Finkelstein JS (2012) Osteoporosis in men: an endocrine society clinical practise guideline. J Clin Endocrinol Metab 97:1802–1822
Guadalupe-Grau A, Fuentes T, Guerra B, Calbet JAL (2009) Exercise and bone mass in adults. Sports Med 39:439–468
Kelley GA, Kelley KS, Tran ZV (2000) Exercise and bone mineral density in men: a meta-analysis. J Appl Physiol 88:1730–1736
Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR (2004) American college of sports medicine position stand: physical activity and bone health. Med Sci Sports Exerc 36:1985–1996
Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, Knipschild PG (1998) The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 51:1235–1241
Verhagen AP, de Vet HC, de Bie RA, Boers M, van den Brandt PA (2001) The art of quality assessment of RCTs included in systematic reviews. J Clin Epidemiol 54:651–654
Woo J, Hong A, Lau E, Lynn H (2007) A randomised controlled trial of Tai Chi and resistance exercise on bone health, muscle strength and balance in community-living elderly people. Age Ageing 262–268
Whiteford J, Ackland TR, Dhaliwal SS, James AP, Woodhouse JJ, Price R, Prince RL, Kerr DA (2010) Effects of a 1-year randomized controlled trial of resistance training on lower limb bone and muscle structure and function in older men. Osteoporos Int 21(9):1529–1536
Kukuljan S, Nowson CA, Bass SL, Sanders K, Nicholson GC, Seibel MJ, Salmon J, Daly RM (2009) Effects of a multi-component exercise programme and calcium-vitamin-D3-fortified milk on bone mineral density in older men: a randomised controlled trial. Osteoporos Int 20(7):1241–1251
Kukuljan S, Nowson CA, Sanders KM, Nicholson GC, Seibel MJ, Salmon J, Daly RM (2011) Independent and combined effects of calcium-vitamin d3 and exercise on bone structure and strength in older men: an 18-month factorial design randomized controlled trial. J Clin Endocrinol Metab 96(4):955–963
Ryan AS, Treuth MS, Rubin MA, Miller JP, Nicklas BJ, Landis DM, Pratley RE, Libanati CR, Gundberg CM, Hurley BF (1994) Effects of strength training on bone mineral density: hormonal and bone turnover relationships. J Appl Physiol 1678–1684
Paillard T, Lafont C, Costes-Salon MC, Rivière D, Dupui P (2004) Effects of brisk walking on static and dynamic balance, locomotion, body composition and aerobic capacity in ageing healthy active men. Int J Sports Med 25(7):539–546
Huuskonen J, Vaisanen SB, Kroger H, Jurvelin JS, Alhava E, Rauramaa R (2001) Regular physical exercise and bone mineral density: a four-year controlled randomized trial in middle-aged men. The DNASCO study. Osteoporos Int 12(5):349–355
Menkes A, Mazel S, Redmond RA, Koffler K, Libanati CR, Gundberg CM, Zizic TM, Hagberg JM, Pratley RE, Hurley BF (1993) Strength training increases regional bone mineral density and bone remodeling in middle-aged and older men. J Appl Physiol 74:2478–2484
Braith RW, Mills RM, Welsch MA, Keller JW, Pollock ML (1996) Resistance exercise training restores bone mineral density in heart transplant recipients. J Am Coll Cardiol 28(6):1471–1477
Turner CH, Robling AG (2005) Mechanisms by which exercise improves bone strength. J Bone Miner Metab 23(Suppl):16–22
Turner CH (1998) Three rules for bone adaptation to mechanical stimuli. Bone 23:399–407
Bailey CA, Brooke-Wavell K (2010) Optimum frequency of exercise for bone health: randomised controlled trial of a high- impact unilateral intervention. Bone 46(4):1043–1049
Martyn-St. James M, Carroll S (2006) High-intensity resistance training and postmenopausal bone loss: a meta-analysis. Osteoporos Int 17:1225–1240
Martyn-St James M, Carroll S (2010) Effects of different impact exercise modalities on bone mineral density in premenopausal women: a meta-analysis. J Bone Miner Metab 28:251–267
Specker B, Vukovich M (2007) Evidence for an interaction between exercise and nutrition for improved bone health during growth. Med Sport Sci 51:50–63
Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, Harbour RT, Caldwell LM, Creed G (2011) Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane database of systematic reviews (Online) CD000333 doi:10.1002/14651858.CD000333.pub2
Martyn-St JM, Carroll S (2008) Meta-analysis of walking for preservation of bone mineral density in postmenopausal women. Bone 43:521–531
Martyn-St JM, Carroll S (2009) A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sports Med 43:898–908
Hunter GR, McCarthy JP, Bamman MM (2004) Effects of resistance training on older adults. Sports Med 34:329–348
Kannus P, Sievanen H, Palvanen M, Jarvinen T, Parkkari J (2005) Prevention of falls and consequent injuries in elderly people. Lancet 366(20):1885–1893
Galvao DA, Nosaka K, Taaffe DR, Spry N, Kristjanson LJ, McGuigan MR, Suzuki K, Yamaya K, Newton RU (2006) Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc 38:2045–2052
Winters-Stone KM, Dobek J, Nail LM, Bennett JA, Leo MC, Torgrimson-Ojerio B, Luoh SW, Schwartz A (2012) Impact + resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: a randomized controlled trial. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA
Saarto T, Sievanen H, Kellokumpu-Lehtinen P et al (2012) Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos Int 23(5):1601–1612
Bolotin HH, Sievanen H (2001) Inaccuracies inherent in dual-energy X-ray absorptiometry in vivo bone mineral density can seriously mislead diagnostic/prognostic interpretations of patient-specific bone fragility. J Bone Miner Res: Off J Am Soc Bone Miner Res 16:799–805
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bolam, K.A., van Uffelen, J.G.Z. & Taaffe, D.R. The effect of physical exercise on bone density in middle-aged and older men: A systematic review. Osteoporos Int 24, 2749–2762 (2013). https://doi.org/10.1007/s00198-013-2346-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-013-2346-1