Abstract
Summary
Increased levels of serum undercarboxylated osteocalcin, which were associated with bone metabolism markers, correlated inversely with indices of glucose metabolism (plasma glucose, hemoglobin A1C, and glycated albumin) in hemodialysis patients with abnormalities of bone metabolism.
Introduction
Undercarboxylated osteocalcin (ucOC), a possible marker of bone metabolism and one of the osteoblast-specific secreted proteins, has recently been reported to be associated with glucose metabolism. We tested the hypothesis that ucOC levels are associated with indices of glucose metabolism in chronic hemodialysis patients with abnormalities of bone metabolism.
Methods
Serum ucOC, bone alkaline phosphatase (BAP, a bone formation marker), and tartrate-resistant acid phosphatase-5b (TRACP-5b, a bone resorption marker) were measured in 189 maintenance hemodialysis patients (96 diabetics and 93 non-diabetics), and their relationships with glucose metabolism were examined.
Results
ucOC correlated positively with BAP (ρ = 0.489, p < 0.0001), TRACP-5b (ρ = 0.585, p < 0.0001) and intact parathyroid hormone (iPTH; ρ = 0.621, p < 0.0001). Serum ucOC levels in the diabetic patients were lower than those of non-diabetic patients (p < 0.001), although there were no significant differences in serum BAP or TRACP-5b between diabetic and non-diabetic patients. Serum ucOC correlated negatively with plasma glucose (ρ = −0.303, p < 0.0001), hemoglobin A1C (ρ = −0.214, p < 0.01), and glycated albumin (ρ = −0.271, p < 0.001), although serum BAP or TRACP-5b did not. In multiple linear regression analysis, log [plasma glucose], log [hemoglobin A1C], and log [glycated albumin] were associated significantly with log [ucOC] after adjustment for age, gender, hemodialysis duration, and body mass index but were not associated with log [BAP], log [TRACP-5b], or log [intact PTH].
Conclusion
Increased levels of serum ucOC, which were associated with bone metabolism markers, were inversely associated with indices of glucose metabolism in hemodialysis patients.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Osteocalcin (OC) is the most abundant non-collagenous protein of bone matrix and is only produced by osteoblasts and odontoblasts under the control of 1,25 dihydroxy-vitamin D and parathyroid hormone (PTH) [1, 2]. γCarboxylation occurs at three glutamine residues in the presence of the cofactor vitamin K, and the carboxylated OC interacts with hydroxyapatite crystals [1]. Undercarboxylated OC (ucOC) has been reported to be associated with a reduced bone mass and a high risk of fracture [3, 4]. OC is released from bone matrix during bone resorption, and increased levels of serum OC are considered to reflect bone resorption, bone formation, or high turnover of bone [1, 5]. OC may play an important role in bone formation, although the function of OC has not yet been fully elucidated [5–7].
Recently, OC, in particular ucOC, has been shown to have a positive effect on both insulin production and insulin sensitivity [8, 9]. Mice lacking OC exhibit decreased β-cell proliferation, glucose intolerance, and insulin resistance [10]. Treatment of wild-type mice with continuously infused recombinant ucOC increases insulin secretion and improves glucose tolerance [11]. Recently, the inhibitory role of decarboxylated OC (ucOC), but not carboxylated OC, in regulating the action of tyrosine phosphatase on insulin secretion and sensitivity has been emphasized [12, 13]. As expected from animal studies, several recent human studies have demonstrated inverse associations between circulating OC, particularly ucOC, and fasting plasma glucose, insulin resistance, and hemoglobin A1C in patients with type 2 diabetes [14–17] and in population-based cohorts [18–20]. However, these human studies have not examined the associations in chronic kidney disease (CKD) with abnormalities of bone metabolism [21]. In patients undergoing maintenance hemodialysis, compared to the remaining population without CKD, several severe abnormalities in bone metabolism (chronic kidney disease-mineral and bone disorder; CKD-MBD), such as secondary hyperparathyroidism, osteomalacia, and adynamic bone disease, have been reported to date [21]. In these states, caused by CKD-MBD, it remains unknown whether ucOC is associated with glucose metabolism.
In the present study, we tested the hypothesis that ucOC levels are associated with indices of glucose metabolism in chronic hemodialysis patients (CKD 5D) with abnormalities of bone metabolism.
Patients and methods
Patients
In 2009, there were 396 maintenance hemodialysis patients at Shirasagi Hospital Kidney Center, who were not admitted to the hospital and had no significant acute illness or malignancy. Of 115 who were diabetic, 96 gave informed consent and were enrolled in the present study. Of the 281 non-diabetic patients, 93 patients who were age- and sex-matched to the above 96 diabetic patients were also enrolled in the present study. All 189 patients (96 diabetics and 93 non-diabetics) gave written informed consent prior to participation in the present study. Underlying kidney diseases of non-diabetic patients were as follows: chronic glomerulonephritis (n = 61), nephrosclerosis (n = 18), polycystic kidney disease (n = 2), other disease (n = 8), and unknown disease (n = 4). None of the patients had a past history of parathyroidectomy or renal transplantation. All patients were free of significant acute illness. They had no past history of fracture or radiographic evidence of vertebral or rib fracture. Past exposure to aluminum was negative in all subjects. The active vitamin D derivatives, calcitriol or alfacalcidol, were given orally to 124 patients. None of the patients received any other medication that might affect calcium or bone metabolism, such as androgen, bisphosphonate, vitamin K or aluminum hydroxide. In the diabetic subgroup, 36 patients were treated with insulin, 38 patients with oral anti-diabetic agents (mainly alpha-glucosidase inhibitors), and 22 patients with diet alone.
Patients underwent hemodialysis 3 times a week in 4–4.5 h sessions using hollow-fiber dialyzer and bicarbonate dialysate containing 3.0 mEq/L calcium. This study was approved by the ethics review committee of the Hospital.
Measurement of serum biochemical parameters of bone metabolism and ucOC
Blood was drawn just before the start of the dialysis session in a non-fasting state. Serum albumin, calcium, phosphorus, and glucose were measured by routine laboratory methods. The serum obtained was stored in aliquots at −20 °C until subsequent assay, with measurements made immediately after thawing. The biochemical parameters for calcium metabolism were determined as described previously [22]. All serum bone markers were measured in the same assay run to avoid inter-assay variance. Serum parathyroid hormone PTH was measured by electrochemiluminescence immunoassay (ECLIA) (Elecsys PTH, Roche Diagnostics GmbH, Mannheim, Germany). The assay detected 5–4,000 pg/ml of intact PTH. The intra- and interassay coefficients of variation were 2.93 % and 3.25 %, respectively. Serum bone alkaline phosphatase (BAP) was measured, as a marker of bone formation, using an enzyme immunoassay (EIA) kit (Alkphase-B; Metra Biosystem, Mountain View, CA) [23, 24]. The assay detected 0.7–140 U/L of BAP. The intra- and interassay coefficients of variation were 2.3 % and 3.1 %, respectively [22]. Serum tartrate-resistant acid phosphatase-5b (TRACP-5b) was measured, as a marker of bone resorption, by fragment absorbed immunocapture enzymatic assay (FAICEA) (Osteolinks TRAP-5b; DS Pharma Biomedical, Osaka, Japan) [25, 26]. The assay detected 10–2,800 mU/dL of TRACP-5b. The intra- and interassay coefficients of variation were 2.15 % and 2.95 %, respectively [27] Serum ucOC concentrations were measured by electrochemiluminescence immunoassay (Picolumi ucOC, Sanko Junyaku, Tokyo, Japan) [17, 28]. The electrochemiluminescence immunoassay for ucOC measures intact ucOC molecule [29]. The assay detected 0.39–50 ng/mL of ucOC [29]. The intra- and interassay coefficients of variation were 2.4 % and 2.9 %, respectively [29]. Glycated albumin was measured by an enzymatic method using the Lucica GA-L kit (Asahi Kasei Pharma Co., Tokyo, Japan). Hemoglobin A1C was measured by routine high performance liquid chromatography, which was standardized according to the Japan Diabetes Society.
Statistical methods
Data are summarized as mean ± standard deviation or median and range as appropriate. Comparisons in mean and median values between the two groups were performed by Student's t test and Mann–Whitney's U test, respectively. Correlations were assessed by non-parametric Spearman's rank correlation test. Multiple linear regression analyses were performed to determine the combined influence of age, gender, hemodialysis duration, body mass index, and intact PTH, BAP, TRACP-5b, and ucOC on the indices of glucose metabolism. Because ucOC, BAP, TRACP-5b, intact PTH, glucose, hemoglobin A1C, and glycated albumin levels exhibited skewed distribution, these variables were entered into multiple linear regression analysis after logarithmic transformation. P values less than 0.05 were considered statistically significant. All calculations were performed on a Windows personal computer using StatView V statistics software (SAS Institute Inc, Cary, NC).
Results
Clinical characteristics of the hemodialysis patients
The clinical characteristics of the diabetic and non-diabetic patients are shown in Table 1. There were no significant differences in age and gender between the diabetic and non-diabetic hemodialysis patients. Hemodialysis duration was significantly shorter for the diabetic patients than for the non-diabetic patients (p < 0.0001), and body weight and body mass index (BMI) was significantly greater in the diabetic patient group (p = 0.0219 and p = 0.0211, respectively). Plasma glucose, hemoglobin A1C, and glycated albumin were significantly higher in the diabetic patient group (p < 0.0001). Intact PTH was significantly lower in the diabetics (p = 0.0188).
Relationship between ucOC and indices of bone metabolism
The median values (range) of the serum ucOC, BAP, and TRACP-5b in all patients were 20.0 (2.2–257.4) ng/mL, 18.8 (8.2–69.2) U/L, and 311 (78–1340) mU/dL, respectively. Relationships between ucOC and indices of bone metabolism are shown in Fig. 1. ucOC correlated significantly and positively with BAP and TRACP-5b (ρ = 0.489, p < 0.0001 and ρ = 0.585, p < 0.0001, respectively). Similarly, ucOC correlated significantly and positively with intact PTH (ρ = 0.621, p < 0.0001).
Relationship between serum undercarboxylated osteocalcin (ucOC) and bone alkaline phosphate (BAP), tartrate-resistant acid phosphate-5b (TRACP-5b), or serum intact PTH. Serum ucOC correlated significantly and positively with serum BAP (a), TRACP-5b (b), and intact PTH (c), using non-parametric Spearman's rank correlation test
Relationship between ucOC and indices of glucose metabolism
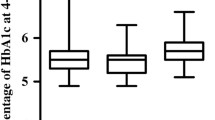
Figure 2 shows ucOC, BAP, and TRACP 5b in non-diabetic and diabetic hemodialysis patients. Serum ucOC levels in the diabetic patients were significantly lower than those of the non-diabetic patients (14.4 (2.7–185.3) vs. 31.5 (2.2–257.4) ng/mL, p < 0.001), although there were no significant differences in serum BAP (18.4 (8.2–69.2) vs. 19.1 (9.1–68.4) U/L, p = 0.5701) and TRACP-5b (291 (87–778) vs. 336 (78–1,340) mU/dL, p = 0.1223) between the diabetic and non-diabetic patients.
Comparison of undercarboxylated osteocalcin (ucOC), bone alkaline phosphate (BAP), and tartrate-resistant acid phosphate-5b (TRACP-5b) between diabetic (DM) and non-diabetic (non-DM) patients. Horizontal bars represent the 10th, 25th, 50th, 75th, and 90th percentile levels. Single asterisk: p < 0.001 vs. non-DM (Mann–Whitney U test)
The relationships between serum ucOC and indices of glucose metabolism were examined in all patients (Fig. 3). Serum ucOC correlated significantly and negatively with plasma glucose (ρ = −0.303, p < 0.0001), hemoglobin A1C (ρ = −0.214, p = 0.0036), and glycated albumin (ρ = −0.271, p = 0.0002). However, serum BAP did not correlate significantly with glucose, hemoglobin A1C, or glycated albumin. Serum TRACP-5b did not correlate significantly with hemoglobin A1C or glycated albumin, although it correlated significantly with plasma glucose (ρ = −0.228, p = 0.0018).
Relationship between undercarboxylated osteocalcin (ucOC) and plasma glucose, hemoglobin A1C (HbA1c) or glycated albumin (GA). Serum ucOC correlated significantly and negatively with plasma glucose (a), HbA1c (b), and GA (c), using non-parametric Spearman's rank correlation test. ρ: Spearman's correlation coefficient by rank
Multiple regression analysis
Multiple linear regression analyses were performed to investigate whether indices of glucose metabolism were associated with serum log [intact PTH], log [BAP], log [TRACP-5b], and log [ucOC] in each model, respectively, after adjustment for age, gender, hemodialysis duration, BMI (Tables 2, 3, and 4). Log [ucOC] was significantly and independently associated with log [plasma glucose] (β = −0.216, p = 0.0063) after adjustment for age, hemodialysis duration, gender, and BMI (R 2 = 0.090, p = 0.0039), but log [intact PTH], log [BAP], or log [TRACP-5b] was not (Table 2). Log [ucOC] was significantly and independently associated with log [hemoglobin A1C] (β = −0.154, p = 0.0436) after adjustment for the same factors (R 2 = 0.142, p < 0.0001), but log [intact PTH], log [BAP], and log [TRACP-5b] were not (Table 3). Log [ucOC] was also significantly and independently associated with log [glycated albumin] (β = −0.176, p = 0.0226) after adjustment for the same factors (R 2 = 0.127, p = 0.0001), but log [intact PTH], log [BAP] or log [TRACP-5b] were not (Table 4).
Discussion
In the present study of chronic hemodialysis patients, we showed that serum ucOC correlated significantly and positively with other bone metabolism markers (i.e., BAP (a bone formation marker), TRACP-5b (a bone resorption marker), and intact PTH), suggesting that ucOC may be indicative of bone turnover status. We showed that, of the three bone metabolism markers of ucOC, BAP, and TRACP-5b, only ucOC was significantly lower in diabetic hemodialysis patients, compared with non-diabetic hemodialysis patients, and that ucOC correlated significantly and inversely with indices of glucose metabolism. The significant association of ucOC with indices of glucose metabolism was demonstrated to be independent of age, hemodialysis duration, gender and BMI.
Hemodialysis patients have a wide variety of bone disorders, including secondary hyperparathyroidism, osteomalacia, and adynamic bone disease, ranging from high turnover of bone to low turnover of bone [21]. Serum bone markers, such as BAP and TRACP, which are not affected by renal function [1, 30], are significantly and positively associated with bone turnover [21]. In the present study examining hemodialysis patients, ucOC was significantly and positively associated with BAP and TRACP, by non-parametric Spearman's rank correlation test. This finding indicated that increased ucOC could be indicative of the status of increased bone turnover. The finding that ucOC correlated significantly and positively with intact PTH in the present study also indicated that patients with high turnover of bone caused by increased PTH may exhibit bone resorption, likely leading to efflux of bone matrix protein of OC into serum and increased concentrations of serum ucOC in these patients.
Concerning OC in diabetic patients, total OC has been shown to be lower in diabetic patients compared with normal subjects [15, 19, 31]. Im et al. [15] reported a significant reduction in serum total OC among type 2 diabetic postmenopausal women compared with normal glucose and impaired fasting glucose groups. Zhou et al. [16] also reported lower serum total OC levels in subjects with newly diagnosed type 2 diabetes, compared with normal glucose tolerance subjects after adjustment for age, gender, and BMI. Kindblom et al. [19] studied 1,010 elderly men and found that diabetic subjects had 21.7 % lower plasma total OC than non-diabetic subjects. Gerdhem et al. have also shown that serum OC levels, but not BAP, were lower in women with diabetes [32]. In the present study, in which hemodialysis patients with abnormalities of bone metabolism were examined, serum ucOC levels were significantly lower in diabetic hemodialysis patients, compared with non-diabetic hemodialysis patients, which is consistent with the previous findings in patients without chronic kidney disease. Because diabetes has been shown to be associated with low turnover of bone in previous studies [14, 32–34], the lower levels of ucOC in diabetics may be considered to be simply due to an association with the low turnover of bone in diabetics. In the present study, however, BAP and TRACP-5b levels were not significantly different between diabetic and non-diabetic hemodialysis patients, suggesting that potential low turnover of bone in diabetic hemodialysis patients does not explain the difference of ucOC levels between diabetic and non-diabetic hemodialysis patients.
Recently, accumulating emerging evidence has suggested that OC correlates with glucose metabolism in both diabetics and non-diabetics. Inverse relationships between total OC and fasting glucose and/or hemoglobin A1C have been confirmed in elderly subjects [18, 20], postmenopausal women [15], newly-diagnosed type 2 diabetes, and normal glucose tolerance subjects [16]. Among those with diabetes, elevated total OC was associated with lower fasting glucose and hemoglobin A1C in 149 postmenopausal women and 179 men [35]. In older Swedish men with and without diabetes, OC correlated negatively with fasting glucose, fasting insulin, and insulin resistance (HOMA-IR) [19]. Recently, the importance of ucOC, not carboxylated OC, on glucose metabolism has been emphasized [12, 13]. Iki et al. [36] reported that in elderly men without diabetes, serum ucOC correlated inversely with fasting plasma glucose, hemoglobin A1C, and insulin resistance (HOMA-IR). In a recent study in patients with type 2 diabetes, ucOC correlated negatively with fasting plasma glucose and hemoglobin A1C levels in men, even after adjustment for age, duration of diabetes, and body stature [17]. In a study of 199 middle-aged men without overt diabetes, elevated serum ucOC was associated with lower fasting and post-challenge glucose levels and higher insulin sensitivity (higher HOMA-β) [37]. None of the above studies, however, examined patients with chronic kidney disease. In the present study, we showed, for the first time, that there was a significant inverse association of ucOC with glucose metabolism indices in hemodialysis patients with abnormalities of bone metabolism. In relationship to bone metabolism, Kanazawa et al. [17] reported that serum ucOC correlated negatively with fasting plasma glucose and hemoglobin A1C in men with type 2 diabetes and normal renal function, but that serum BAP and urinary N-terminal cross-linked telopeptide of type-I collagen (a bone resorption marker) did not correlate with any parameter related to glucose metabolism. In the present study of hemodialysis patients with abnormalities of bone metabolism, we demonstrated that plasma glucose, hemoglobin A1C, and glycated albumin correlated significantly and negatively with ucOC in multiple regression analyses, but not with BAP, TRACP-5b, or intact PTH.
There are some limitations in this study. First, the sample size was relatively small. Second, the cross-sectional nature of the analysis did not allow for interpretation of causality regarding the relationship between serum ucOC and glycemic control. Third, we measured ucOC using ELISA. As such, further studies should use the hydroxyapatite binding assay to compare OC, ucOC, and the ratio ucOC/OC with glycemic control. Fourth, non-fasting serum glucose is of limited value for glycemic control. Fifth, in the present study, we did not measure bone status, such as bone density or bone quality, which might provide useful additional information on the relationship between ucOC and bone status. However, our cross-sectional study demonstrated a distinct association of ucOC, a possible marker of bone metabolism, with glycemic control, such as hemoglobin A1C, glycated albumin, and non-fasting serum glucose concentration, in hemodialysis patients.
In conclusion, we demonstrated, for the first time, that indices of glucose metabolism in chronic hemodialysis patients with abnormalities of bone metabolism correlated significantly and inversely with ucOC, which, in turn, correlated significantly with bone metabolism markers of BAP and TRACP-5b. These findings suggest that ucOC has a distinct association with glucose metabolism, in addition to being an index of bone markers in hemodialysis patients.
References
Urena P, De Vernejoul MC (1999) Circulating biochemical markers of bone remodeling in uremic patients. Kidney Int 55:2141–2156
Yu XP, Chandrasekhar S (1997) Parathyroid hormone (PTH 1–34) regulation of rat osteocalcin gene transcription. Endocrinology 138:3085–3092
Szulc P, Chapuy MC, Meunier PJ, Delmas PD (1993) Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J Clin Invest 91:1769–1774
Szulc P, Arlot M, Chapuy MC, Duboeuf F, Meunier PJ, Delmas PD (1994) Serum undercarboxylated osteocalcin correlates with hip bone mineral density in elderly women. J Bone Miner Res 9:1591–1595
Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J (2000) The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos Int 11(Suppl 6):S2–S17
Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G (1996) Increased bone formation in osteocalcin-deficient mice. Nature 382:448–452
Nickolas TL, Cremers S, Zhang A, Thomas V, Stein E, Cohen A, Chauncey R, Nikkel L, Yin MT, Liu XS, Boutroy S, Staron RB, Leonard MB, McMahon DJ, Dworakowski E, Shane E (2011) Discriminants of prevalent fractures in chronic kidney disease. J Am Soc Nephrol 22:1560–1572
Motyl KJ, McCabe LR, Schwartz AV (2010) Bone and glucose metabolism: a two-way street. Arch Biochem Biophys 503:2–10
Ng KW (2011) Regulation of glucose metabolism and the skeleton. Clin Endocrinol (Oxf) 75:147–155
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469
Ferron M, Hinoi E, Karsenty G, Ducy P (2008) Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A 105:5266–5270
Confavreux CB (2011) Bone: from a reservoir of minerals to a regulator of energy metabolism. Kidney Int Suppl 121:S14–S19
Clemens TL, Karsenty G (2011) The osteoblast: an insulin target cell controlling glucose homeostasis. J Bone Miner Res 26:677–680
Dobnig H, Piswanger-Solkner JC, Roth M, Obermayer-Pietsch B, Tiran A, Strele A, Maier E, Maritschnegg P, Sieberer C, Fahrleitner-Pammer A (2006) Type 2 diabetes mellitus in nursing home patients: effects on bone turnover, bone mass, and fracture risk. J Clin Endocrinol Metab 91:3355–3363
Im JA, Yu BP, Jeon JY, Kim SH (2008) Relationship between osteocalcin and glucose metabolism in postmenopausal women. Clin Chim Acta 396:66–69
Zhou M, Ma X, Li H, Pan X, Tang J, Gao Y, Hou X, Lu H, Bao Y, Jia W (2009) Serum osteocalcin concentrations in relation to glucose and lipid metabolism in Chinese individuals. Eur J Endocrinol 161:723–729
Kanazawa I, Yamaguchi T, Yamauchi M, Yamamoto M, Kurioka S, Yano S, Sugimoto T (2011) Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int 22:187–194
Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B (2009) Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab 94:827–832
Kindblom JM, Ohlsson C, Ljunggren O, Karlsson MK, Tivesten A, Smith U, Mellstrom D (2009) Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res 24:785–791
Yeap BB, Chubb SA, Flicker L, McCaul KA, Ebeling PR, Beilby JP, Norman PE (2010) Reduced serum total osteocalcin is associated with metabolic syndrome in older men via waist circumference, hyperglycemia, and triglyceride levels. Eur J Endocrinol 163:265–272
(2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113:S1–S130
Okuno S, Inaba M, Kitatani K, Ishimura E, Yamakawa T, Nishizawa Y (2005) Serum levels of C-terminal telopeptide of type I collagen: a useful new marker of cortical bone loss in hemodialysis patients. Osteoporos Int 16:501–509
Gomez B Jr, Ardakani S, Ju J, Jenkins D, Cerelli MJ, Daniloff GY, Kung VT (1995) Monoclonal antibody assay for measuring bone-specific alkaline phosphatase activity in serum. Clin Chem 41:1560–1566
Kumeda Y, Inaba M, Tahara H, Kurioka Y, Ishikawa T, Morii H, Nishizawa Y (2000) Persistent increase in bone turnover in Graves' patients with subclinical hyperthyroidism. J Clin Endocrinol Metab 85:4157–4161
Nishizawa Y, Inaba M, Ishii M, Yamashita H, Miki T, Goto H, Yamada S, Chaki O, Kurasawa K, Mochizuki Y (2008) Reference intervals of serum tartrate-resistant acid phosphatase type 5b activity measured with a novel assay in Japanese subjects. J Bone Miner Metab 26:265–270
Ohashi T, Igarashi Y, Mochizuki Y, Miura T, Inaba N, Katayama K, Tomonaga T, Nomura F (2007) Development of a novel fragments absorbed immunocapture enzyme assay system for tartrate-resistant acid phosphatase 5b. Clin Chim Acta 376:205–212
Shidara K, Inaba M, Okuno S, Yamada S, Kumeda Y, Imanishi Y, Yamakawa T, Ishimura E, Nishizawa Y (2008) Serum levels of TRAP5b, a new bone resorption marker unaffected by renal dysfunction, as a useful marker of cortical bone loss in hemodialysis patients. Calcif Tissue Int 82:278–287
Kanazawa I, Yamaguchi T, Yamauchi M, Yamamoto M, Kurioka S, Yano S, Sugimoto T (2009) Adiponectin is associated with changes in bone markers during glycemic control in type 2 diabetes mellitus. J Clin Endocrinol Metab 94:3031–3037
Nishimura J, Arai N, Tohmatsu J (2007) Measurement of serum undercarboxylated osteocalcin by ECLIA with the “Picolumi ucOC” kit. Clin Calcium 17:1702–1708 (in Japanese)
Yamada S, Inaba M, Kurajoh M, Shidara K, Imanishi Y, Ishimura E, Nishizawa Y (2008) Utility of serum tartrate-resistant acid phosphatase (TRACP5b) as a bone resorption marker in patients with chronic kidney disease: independence from renal dysfunction. Clin Endocrinol (Oxf) 69:189–196
Inaba M, Nishizawa Y, Mita K, Kumeda Y, Emoto M, Kawagishi T, Ishimura E, Nakatsuka K, Shioi A, Morii H (1999) Poor glycemic control impairs the response of biochemical parameters of bone formation and resorption to exogenous 1,25-dihydroxyvitamin D3 in patients with type 2 diabetes. Osteoporos Int 9:525–531
Gerdhem P, Isaksson A, Akesson K, Obrant KJ (2005) Increased bone density and decreased bone turnover, but no evident alteration of fracture susceptibility in elderly women with diabetes mellitus. Osteoporos Int 16:1506–1512
Martin KJ, Gonzalez EA (2007) Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol 18:875–885
Malluche HH, Monier-Faugere MC (1992) Risk of adynamic bone disease in dialyzed patients. Kidney Int Suppl 38:S62–S67
Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Kurioka S, Yano S, Sugimoto T (2009) Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab 94:45–49
Iki M, Tamaki J, Fujita Y, Kouda K, Yura A, Kadowaki E, Sato Y, Moon JS, Tomioka K, Okamoto N, Kurumatani N (2011) Serum undercarboxylated osteocalcin levels are inversely associated with glycemic status and insulin resistance in an elderly Japanese male population: Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Study. Osteoporos Int 23:761–770
Hwang YC, Jeong IK, Ahn KJ, Chung HY (2009) The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced beta-cell function in middle-aged male subjects. Diabetes Metab Res Rev 25:768–772
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okuno, S., Ishimura, E., Tsuboniwa, N. et al. Significant inverse relationship between serum undercarboxylated osteocalcin and glycemic control in maintenance hemodialysis patients. Osteoporos Int 24, 605–612 (2013). https://doi.org/10.1007/s00198-012-2003-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-012-2003-0