Abstract
Summary
Hydrofluoric etching and anodized hierarchical micro/nanotextured surface titanium implant was placed in mandibles of ovariectomized sheep for 12 weeks, and it showed improved osseointegration by resonance frequency analysis (RFA), microcomputed tomography (micro-CT) evaluation, histomorphometry, and biomechanical test.
Introduction
This study aimed to investigate the effects of micro/nanotextured titanium implant on osseointegration in ovariectomized (OVX) sheep.
Methods
The hierarchical micro/nanotextured surface of titanium implant was fabricated by acid in 0.5% (w/v) hydrofluoric (HF) and anodized in HF acid electrolytes with a DC power of 20 V, and the machined surface implants with no treatment served as control group. The implants were placed in mandibles of OVX sheep, respectively. Twelve weeks after implantation, RFA, microcomputed tomography, histomorphometry, and biomechanical tests were applied to detect the osseointegration of the two groups.
Results
The implant stability quotient (ISQ) values, the maximum pull-out forces, and the bone–implant contact (BIC) were 65.5 ± 6.3, 490.6 ± 72.7 N, and 58.31 ± 5.79% in the micro/nanogroup and 58.3 ± 8.9, 394.5 ± 54.5 N, and 46.85 ± 5.04% in the control group, respectively. There was no significant difference between the two groups in ISQ values (p > 0.05), but in the micro/nanogroup, the maximal pull-out force and the BIC were increased significantly (p < 0.05 or p < 0.01). Micro-CT analysis showed that the bone volume ratio and the trabecular number increased significantly (p < 0.01), and the trabecular separation decreased significantly (p < 0.05) in the micro/nanogroup.
Conclusion
Implant modification by HF acid etching and anodization to form a hierarchical micro/nanotextured surface could improve titanium implant osseointegration in OVX sheep 12 weeks after implantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prosthetic treatment with titanium implant has brought comfort and esthetic results to patients and therefore earned a favorable reputation from both patients and dentists [1]. Whether an implant will be successful or not is determined by many factors, among which bone quality and quantity are extremely important [2]. Poor bone quality, especially low bone density, has been pointed out as a major reason that would prolong bone–implant healing period or even cause implant failure [3]. The reduction of bone mass often occurs in elders who suffer from a retrogression process called osteoporosis [4]; moreover, this same group of people also often suffers from edentulous condition with osteoporotic jawbones and needs reconstruction with well mastication [5]. Although no data contraindicating dental implants have been reported in osteoporotic patients, lower osseointegration rates in osteoporotic bone than in normal bone have been reported [6, 7], and a proper adjustment of surgical techniques, a longer healing period, and implant modification may be considered in order to achieve better osseointegration [8].
Physicochemical characteristics of the dental implant surface, such as roughness, topography, chemistry, and electrical charge, affect the biological reactions occurring at the interface of tissue and implant [9]. There were observations that a micron-scale rough surface prepared by grit blasting and subsequent acid etching was capable of rapid and increased bone accrual at titanium implants [10]. Novel nanostructures at implant surface created by chemical treatments (peroxidation (H2O2) or acid oxidation, such as hydrofluoric acid) have also been shown favorable bioactivities with titanium surfaces [11]. It was reported that the fluoride-modified implants showed increased surface roughness at the nanolevel and improved titanium implant osseointegration in osteoporotic bones [12]. Natural tissues are hierarchical structures assembled in a highly organized way composed of nano-, micro-, and macroscale building blocks, of which bone tissues constitute a good example [13]. There have been successful attempts to fabricate such micro/nanostructures in implant surfaces modifications. In an in vitro study, the micropit-and-nanonodule hybrid titania topography on the titanium surface showed enhanced attachment, spreading, adhesion, proliferation, and differentiation of osteoblasts [14]. Our previous study indicated that the hierarchical micro/nanotextured surfaces lead to enhancement of faster bone maturation around the titanium implants without compromising the bone mass, and the hierarchical micro/nanotextured surfaces still retain the mechanical interlocking ability of the microtopography thereby boding well for osseointegration [15]. However, few studies about the in vivo effects of hierarchical micro/nanotextured surfaces on bone–implant osseointegration have been reported, especially in osteoporosis.
The purpose of this preliminary study was to evaluate the effects of hierarchical micro/nanotextured surface implant on osseointegration of ovariectomized sheep and to find its utility in osteoporosis.
Materials and methods
Implant and surface modification
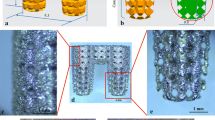
Thirty-two titanium cylindrical screwed machined surface implants (4.3 × 10 mm; Zhongbang Corporation, Xi’an, China; Fig. 1a) were used. Sixteen implants were cleaned by ultrasonic cleaning, and then the samples were treated with 0.5% (w/v) hydrofluoric (HF) acid for 30 min. Immediately after acid treatment, they were rinsed with distilled water and dried and were anodized for 30 min in an electrolyte containing 0.5% (w/v) HF acid using a DC power (20 V) supply with a platinum electrode as the cathode [15]. Immediately after anodization, they were rinsed with deionized water and then sterilized in an autoclave. The other 16 implants were kept non-modified as control group. Field-emission scanning electron microscopy (SEM; JSM-6700F, JEOL) was utilized to observe the hierarchical micro/nanotextured surface topography of the samples.
Implant surface manifestation. a Schematic view of Zhongbang®-machined surfaced titanium implants; b the implant after surface modification by HF acid and anodization; c the SEM view (amplified to 30,000) of implant surface after HF acid; d, e the SEM view of implant surface after HF acid and anodization (amplified to 30,000 and 100,000)
Animals and surgical procedures
The animal experiments were conducted according to the Guidelines for the Care and Use of Laboratory Animals as well as being compliant with the Animal Research Committee of the University. Eight sheep (age, 4 ± 0.9 years old; mean weight, 45 ± 4 kg) were included in this study, and the bilateral ovaries were removed (ovariectomy, OVX). Bone mineral density (BMD) was measured using a dual energy X-ray absorption meter (Lunar Corp., Madison, WI, USA) at baseline (pre-operation) and 12 months after bilateral OVX [16]. BMD of the lumbar spine and mandibular angle were obtained to ensure that the animals were osteoporotic (Fig. 2). Then implantation were applied at the bilateral mandibular angle (Fig. 3), with the left side using micro/nanosurfaced implants while the right side using the controls. These operations were carried out with the animals under general anesthesia using sodium pentobarbital (30 mg/kg, China) by intramuscular injection. Postoperatively, antibiotics (ampicillin, 12.5 mg/kg, China) were administered for 5 days. All animals were housed indoors under identical conditions. Twelve weeks after implantation surgery, all the animals were killed and the mandibles with implants were harvested for the following evaluation.
Resonance frequency analysis
Resonance frequency analysis was recorded as implant stability quotient (ISQ) by means of an Osstell machine (Integration Diagnostics AB, Gothenburg, Sweden). With a transducer connected into the implant abutment, it was measured on samples of both groups 12 weeks after implantation.
Biomechanical test
Immediately after specimen harvest, half of the specimens in each group were evaluated by biomechanical pull-out test, using a universal material testing system (AGS-10KNG, Shimadzu, Japan). The pull-out test was performed at a compression speed of 5 mm/min. Displacement versus force was recorded and used for the determination of the maximal pull-out force (Fig. 4).
Histological analysis
The other half of the specimens in each group were maintained in 10% buffered formalin for 2 days. Each specimen was dehydrated through a graded series of ethanol solutions and 100% acetone. Subsequently, the bone samples were embedded in polyester resin and mounted in a sawing microtome (Leica SP 1600, Leica Microsystems, Wetzlar, Germany). A 30-μm-thick undecalcified section was obtained, which then was stained with Ponceau trichrome stain.
Histometric analysis was performed by means of a computer-digitized image analysis system (Leica Imaging System, Cambridge, England) coupled to a light microscope (Olympus BH2 with S Plan FL2 lens, Tokyo, Japan) with a high-resolution video camera (CDC/RGB color video camera, Sony, Fujisawa, Japan) and a Sony Trinitron monitor (image resolution, 276 512 × 512; Fig. 5). Bone–implant contact (BIC) was calculated as the linear percentage of the interface with direct bone-to-implant contact to total interface of the implant in the cancellous bone.
Ponceau trichrome stains of the undecalcified sections after 12 weeks of osseointegration (a, b transverse planes; c, d axis planes, ×25 magnification). a, c The micro/nanosurfaced group with more new bone formation adherent to the implant surface tightly; c, d the control group with less new bone formation and poor osseointegration around the implants
Micro-CT evaluation
Before being applied to mechanical test and dehydrated in histological analysis, the specimens were scanned on micro-CT (Inveon, Siemens, Germany; 80 kV, 500 mA, 800 ms integration time) and reconstructed with an isotropic voxel size of 10 mm. The three-dimensional images acquired from microtomographic slices were utilized for quantitative evaluation. The regions of interest including the trabecular compartment around implant were selected, which was defined as a ring with a radius of 2 mm from the implant surface [12] (Fig. 6c). Trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp), bone surface/bone volume (BS/BV), and bone volume/total volume (BV/TV) were determined.
Statistical analysis
Data analysis was carried out with SPSS 13.0 software (SPSS, Chicago, IL, USA). Multiple comparisons between groups were performed using t test; the data were expressed as means, with indication of the standard deviation (SD) of the mean (mean ± SD). Significant differences were considered as p < 0.05 and highly considered when p < 0.01.
Results
Surface topography of the hierarchical micro/nanotextured implant
The microstructured surface was formed initially on the titanium surface by etching in 0.5% HF acid, and the nanotubular layers fabricated on the microsurface by anodization were illustrated in Fig. 1. After subsequent anodization, the surface structure was smoothed, and nanotubes of different sizes (diameters of about 15 and 80 nm) formed on the microstructured surface distributed quite uniformly.
BMD results of the animal
Twelve months after OVX, BMD of lumbar spine and the mandibular angle showed significant decrease from baseline value (pre-OVX BMD; p < 0.01; Table 1), indicating that the osteoporotic animal model was successfully established.
ISQ measurement and biomechanical test
After 12 weeks of implantation, the results of ISQ values and the maximal pull-out force were shown in Table 2. There was no significant difference between the micro/nanogroup and the control group in ISQ values (p > 0.05), but the maximal pull-out force significantly increased in the micro/nanogroup (p < 0.05).
Histological analysis
The implants and osseointegration of peri-implant trabecular bones were showed in the undecalcified sections with Ponceau trichrome stain. The calcified bone in the stained slides (both the axis and transverse planes) was distinguished from other tissues by its characteristic color (Fig. 5). An average of three sections in each specimen was used for analysis, and the results of histomorphometry were expressed as BIC. In 12 weeks after implantation, micro/nanomodified implants showed significantly increased BIC compared to control (p < 0.01) (Table 2).
Micro-CT evaluation
The micro-CT images through the center of implant axis were showed in Fig. 6, which clearly depicted the osseointegration of implants with the trabecular bones in the two groups. Compared with control implants (Table 3), the BV/TV and the Tb.N increased significantly in the micro/nanogroup (p < 0.01), and the Tb.Sp decreased significantly (p < 0.05). No statistically significant changes were found in Tb.Th and BS/BV values between the two groups.
Discussion
Under osteoporosis, both bone formation and maturation were delayed [17], and decrease of BIC was observed in animals with osteoporosis-like conditions [6, 18]. In this study, the BIC of the hierarchical micro/nanosurface implants were significantly higher than the control. The result was in accordance with a recent study in which it was shown that the fluoride-modified implant had a higher osseointegration and promoted peri-implant new bone formation compared to control group in osteoporosis [12]. The ISQ measurement between the micro/nanogroup and the control was not significant in this study. This may be due to the reason that the factors affected RFA on dental implant are the rigidity of surrounding tissues, which is mainly affected by cortical bones [19]. It was also shown that the osseointegration of cortical bones of the two groups was with no obvious distinction in the micro-CT images (Fig. 6). The biomechanical test of the maximum pull-out force result showed better mechanical characteristics in the hierarchical micro/nanogroup, which was similar with previous studies [20, 21]. The micro-CT analysis in BV/TV, Tb.N, and the Tb.Sp differences indicated that the micro/nanosurface implant had a better effect in osseointegration of osteoporotic jawbone.
Although the diagnosis of osteoporosis and osteopenia did not contribute to the increased risk of implant failure [22, 23], a prolonged healing period is required to achieve the best possible secondary stability in osteoporotic conditions [24, 25]. Alsaadi et al. have reported a significant association between osteoporosis with early implant failures [26]. It has been shown that mandibular osteoporosis prior to implant treatment may be a risk factor for bone loss around implants with implant-supported overdentures [27]. Due to the inferiority of bone quality and quantity caused by osteoporosis, the support and retention of the bone to the implant were weakened. Therefore, the load-bearing implants in osteoporotic bones with thin cortices and reduced spongiosa require larger areas to provide more load-bearing bone–implant interfaces. Previous studies about applying implant under osteoporosis emphasized medication treatment. However, the medicine usage should be long-lasting and its long-term effects were not determined [28, 29]. The modified titanium surface fabricated by acid or oxidation method transformed from the inside of the metal base, which was expected to promote bone–implant contact via mechanical interlocks and provide with integration more tightly to metal base [30]. Particularly, the hierarchical micro/nanotopographies would provide larger surface for bone–implant osseointegration. The increase of surface roughness of implants at the nanolevel might contribute to the promoted new bone formation and then the improved implant osseointegration.
Micro- or nanosurface modification of titanium implant offers the advantage of improving surface biocompatibility between the bones and implant surface with favorable osseointegration. Improved implant osseointegration in mandible or tibia of dogs was demonstrated at the acid-etched implants surface modified on the microscale [31], and implants modified with 0.01% HF showed the highest retention in tibia of rat [21]. The topography of titanium surface at nanolevel has been reported to modulate differentiation, proliferation, and gene expression of osteoblasts and osteoblast-like cells at intrinsic part of the clinical performance of this surface [20]. A recent study indicated that HF treatment of titanium surface with nanostructures improved implant osseointegration in OVX rats and suggested the feasibility of using fluoride modification to improve titanium implant osseointegration in osteoporotic bone [12]. Hierarchical micro/nanostructures were identified to have biocompatible contributions to bone formation on the titanium surface. Gao et al. fabricated a micro/nanostructured porous surface on titanium and observed increased hydroxyapatite formation [32], and Kubo [14] and Zinger [33] found that microscale and nanoscale topography of the implant surface exhibited a synergistic effect on bone marrow osteoblasts and human-bone-derived MG63 cells proliferation. Furthermore, Park et al. showed that the nanostructured Ca-incorporated oxide layer produced by hydrothermal treatment on microstructured titanium implants surface was effective in improving the osseointegration of implants in areas of loose cancellous bone [34].
The effects of the micro/nanostructure titanium surface on implant osseointegration have rarely been reported in osteoporotic bone conditions. The present study found that apparently higher osseointegration was achieved in the micro/nanostructure titanium surface implant than the control group. After HF acid etching and anodization, the increase of surface roughness at the micro/nanolevel might contribute to the improved implant osseointegration in osteoporosis. Previous studies found that the changes of surface chemical characteristics also played an important role in the improved biocompatibility of implants; for example, the fluoride-modified titanium exhibited increased content of titanium, oxygen, and fluorine and decreased content of carbon on the surface which has been speculated to contribute to improved biocompatibility [21, 35]. In addition, fluoride ions on titanium surface after HF treatment have been shown to increase osteoblast differentiation and gene expression [20, 35]. Commonly and easily, the hierarchical micro/nanotextured surface topographies in this study were produced on titanium using HF acid etching combined with anodization, so it was a simple, convenient, and practical method.
Conclusions
The results of this study suggested the feasibility of modifying implant surface by using HF acid etching and anodization to form a hierarchical micro/nanotextured surface which could improve titanium implant osseointegration in osteoporotic jawbone of ovariectomized sheep 12 weeks after implantation. However, longer healing period studies and further mechanism exploration studies are necessary to develop a better knowledge about this hierarchical micro/nanotextured surface titanium implant in osteoporosis.
References
Ekelund JA, Lindquist LW, Carlsson GE, Jemt T (2003) Implant treatment in the edentulous mandible: a prospective study on Branemark system implants over more than 20 years. Int J Prosthodont 16:602–608
Goiato MC, Pellizzer EP, dos Santos DM, Barao VA, de Carvalho BM, Magro-Filho O, Garcia IR Jr (2009) Clinical viability of immediate loading of dental implants: part I—factors for success. J Craniofac Surg 20:2139–2142
Alsaadi G, Quirynen M, Komarek A, van Steenberghe D (2008) Impact of local and systemic factors on the incidence of late oral implant loss. Clin Oral Implants Res 19:670–676
Curran D, Maravic M, Kiefer P, Tochon V, Fardellone P (2010) Epidemiology of osteoporosis-related fractures in France: a literature review. Joint Bone Spine. doi:10.1016/j.jbspin.2010.02.006
Deguchi T, Yoshihara A, Hanada N, Miyazaki H (2008) Relationship between mandibular inferior cortex and general bone metabolism in older adults. Osteoporos Int 19:935–940
Keller JC, Stewart M, Roehm M, Schneider GB (2004) Osteoporosis-like bone conditions affect osseointegration of implants. Int J Oral Maxillofac Implants 19:687–694
Yildiz A, Esen E, Kurkcu M, Damlar I, Daglioglu K, Akova T (2010) Effect of zoledronic acid on osseointegration of titanium implants: an experimental study in an ovariectomized rabbit model. J Oral Maxillofac Surg 68:515–523
Tsolaki IN, Madianos PN, Vrotsos JA (2009) Outcomes of dental implants in osteoporotic patients. A literature review. J Prosthodont 18:309–323
Kim TI, Jang JH, Kim HW, Knowles JC, Ku Y (2008) Biomimetic approach to dental implants. Curr Pharm Des 14:2201–2211
Mendonca G, Mendonca DB, Aragao FJ, Cooper LF (2008) Advancing dental implant surface technology—from micron- to nanotopography. Biomaterials 29:3822–3835
Wang XX, Hayakawa S, Tsuru K, Osaka A (2001) A comparative study of in vitro apatite deposition on heat-, H(2)O(2)-, and NaOH-treated titanium surfaces. J Biomed Mater Res 54:172–178
Li Y, Zou S, Wang D, Feng G, Bao C, Hu J (2010) The effect of hydrofluoric acid treatment on titanium implant osseointegration in ovariectomized rats. Biomaterials 31:3266–3273
Rho JY, Kuhn-Spearing L, Zioupos P (1998) Mechanical properties and the hierarchical structure of bone. Med Eng Phys 20:92–102
Kubo K, Tsukimura N, Iwasa F, Ueno T, Saruwatari L, Aita H, Chiou WA, Ogawa T (2009) Cellular behavior on TiO2 nanonodular structures in a micro-to-nanoscale hierarchy model. Biomaterials 30:5319–5329
Zhao L, Mei S, Chu PK, Zhang Y, Wu Z (2010) The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions. Biomaterials 31:5072–5082
Wu ZX, Lei W, Hu YY, Wang HQ, Wan SY, Ma ZS, Sang HX, Fu SC, Han YS (2008) Effect of ovariectomy on BMD, micro-architecture and biomechanics of cortical and cancellous bones in a sheep model. Med Eng Phys 30:1112–1118
Fini M, Giavaresi G, Rimondini L, Giardino R (2002) Titanium alloy osseointegration in cancellous and cortical bone of ovariectomized animals: histomorphometric and bone hardness measurements. Int J Oral Maxillofac Implants 17:28–37
Cho P, Schneider GB, Krizan K, Keller JC (2004) Examination of the bone–implant interface in experimentally induced osteoporotic bone. Implant Dent 13:79–87
Pattijn V, Jaecques SV, De Smet E, Muraru L, Van Lierde C, Van der Perre G, Naert I, Vander Sloten J (2007) Resonance frequency analysis of implants in the guinea pig model: influence of boundary conditions and orientation of the transducer. Med Eng Phys 29:182–190
Monjo M, Lamolle SF, Lyngstadaas SP, Ronold HJ, Ellingsen JE (2008) In vivo expression of osteogenic markers and bone mineral density at the surface of fluoride-modified titanium implants. Biomaterials 29:3771–3780
Lamolle SF, Monjo M, Lyngstadaas SP, Ellingsen JE, Haugen HJ (2009) Titanium implant surface modification by cathodic reduction in hydrofluoric acid: surface characterization and in vivo performance. J Biomed Mater Res A 88:581–588
Holahan CM, Koka S, Kennel KA, Weaver AL, Assad DA, Regennitter FJ, Kademani D (2008) Effect of osteoporotic status on the survival of titanium dental implants. Int J Oral Maxillofac Implants 23:905–910
Amorim MA, Takayama L, Jorgetti V, Pereira RM (2007) Comparative study of axial and femoral bone mineral density and parameters of mandibular bone quality in patients receiving dental implants. Osteoporos Int 18:703–709
Mori H, Manabe M, Kurachi Y, Nagumo M (1997) Osseointegration of dental implants in rabbit bone with low mineral density. J Oral Maxillofac Surg 55:351–361
Friberg B, Ekestubbe A, Mellstrom D, Sennerby L (2001) Branemark implants and osteoporosis: a clinical exploratory study. Clin Implant Dent Relat Res 3:50–56
Alsaadi G, Quirynen M, Komarek A, van Steenberghe D (2007) Impact of local and systemic factors on the incidence of oral implant failures, up to abutment connection. J Clin Periodontol 34:610–617
von Wowern N, Gotfredsen K (2001) Implant-supported overdentures, a prevention of bone loss in edentulous mandibles? A 5-year follow-up study. Clin Oral Implants Res 12:19–25
Bornstein MM, Cionca N, Mombelli A (2009) Systemic conditions and treatments as risks for implant therapy. Int J Oral Maxillofac Implants 24(Suppl):12–27
Madrid C, Sanz M (2009) What impact do systemically administrated bisphosphonates have on oral implant therapy? A systematic review. Clin Oral Implants Res 20(Suppl 4):87–95
Ma W, Wei JH, Li YZ, Wang XM, Shi HY, Tsutsumi S, Li DH (2008) Histological evaluation and surface componential analysis of modified micro-arc oxidation-treated titanium implants. J Biomed Mater Res B Appl Biomater 86:162–169
Daugaard H, Elmengaard B, Bechtold JE, Soballe K (2008) Bone growth enhancement in vivo on press-fit titanium alloy implants with acid etched microtexture. J Biomed Mater Res A 87:434–440
Gao L, Feng B, Wang J, Lu X, Liu D, Qu S, Weng J (2009) Micro/nanostructural porous surface on titanium and bioactivity. J Biomed Mater Res B Appl Biomater 89B:335–341
Zinger O, Anselme K, Denzer A, Habersetzer P, Wieland M, Jeanfils J, Hardouin P, Landolt D (2004) Time-dependent morphology and adhesion of osteoblastic cells on titanium model surfaces featuring scale-resolved topography. Biomaterials 25:2695–2711
Park JW, Kim HK, Kim YJ, An CH, Hanawa T (2009) Enhanced osteoconductivity of micro-structured titanium implants (XiVE S CELLplus) by addition of surface calcium chemistry: a histomorphometric study in the rabbit femur. Clin Oral Implants Res 20:684–690
Lamolle SF, Monjo M, Rubert M, Haugen HJ, Lyngstadaas SP, Ellingsen JE (2009) The effect of hydrofluoric acid treatment of titanium surface on nanostructural and chemical changes and the growth of MC3T3-E1 cells. Biomaterials 30:736–742
Acknowledgment
This work was supported by grants from the nature Science Foundation of China (project no. 30900284).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jianrui Xiao and Hong Zhou equally contributed to this study.
Rights and permissions
About this article
Cite this article
Xiao, J., Zhou, H., Zhao, L. et al. The effect of hierarchical micro/nanosurface titanium implant on osseointegration in ovariectomized sheep. Osteoporos Int 22, 1907–1913 (2011). https://doi.org/10.1007/s00198-010-1413-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-010-1413-0