Abstract
Summary
Fall prevention is a key strategy for reducing osteoporotic fractures. We investigated the association between vitamin D receptor (VDR) polymorphisms and reported falls in postmenopausal women. Bsm1 polymorphisms were associated with falls, balance and muscle power measurements. These results may explain some of the excess fracture risk associated with VDR in some studies.
Introduction
Fall prevention is a key strategy for reducing osteoporotic fractures. It has been suggested that vitamin D supplementation may reduce the incidence of falls by reducing body sway and increasing muscle power. The vitamin D receptor gene is a well-studied candidate gene for osteoporosis. We investigated the association between VDR polymorphisms and reported falls in postmenopausal women.
Methods
Falls data were collected in two separate population cohorts. Five polymorphisms of the VDR gene were analysed (Cdx-2, Fok-1, BsmI, Taq1 and Apa1) in the Aberdeen Prospective Osteoporosis Screening Study (APOSS) cohort. Results found in APOSS were then validated in an independent cohort—the Osteoporosis and Ultrasound (OPUS) study (Bsm1 and Fok1 only), where muscle power and balance were also measured.
Results
Carriers of the ‘B’ allele (Bsm1) showed an increased risk for falls. In APOSS, this was statistically significant for visit 3 multiple falls (p = 0.047) and for recurrent falls (p = 0.043). Similar results were found in OPUS for visit 1 falls (p = 0.025) and visit 1 multiple falls (p = 0.015). Bsm1 polymorphisms were also associated with balance and muscle power measurements.
Conclusions
In conclusion, these results demonstrate an association between the Bsm1 polymorphism and risk of falling that may explain some of the excess fracture risk associated with VDR in some studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporotic fracture is a major cause of morbidity and mortality in elderly men and women. While it is recognised that the major measurable risk factor for fracture in post-menopausal women is low bone mineral density, there is increasing realisation that this does not explain all osteoporotic fractures. Hence, the role of other risk factors, including fall-related aspects, which have an independent effect on fracture occurrence, is receiving increasing interest [1].
The number of falls increases with age [2–5] with approximately 30% of the free-living elderly population falling each year [6]. Up to 90% or more of hip fractures occur following a fall [7].
Risk factors for falls are numerous but include visual impairment, use of specific drugs, such as hypnotics, sedatives and diuretics, chronic conditions including arthritis and Parkinson’s disease [3, 8]. Impaired balance and increased body sway have been also shown to be important causes of falls [5, 9, 10], suggesting a role for decreased muscle power. Recent meta-analyses suggest a significant reduction in fall rates of between 12% and 22% when individuals are treated with vitamin D supplementation [11, 12]. The mechanism of action appears to be acting by a reduction in body sway [13] and this, plus an improvement in lower body strength, may reduce the number of falls and hence fractures [14].
Many studies have been performed to date studying the association between candidate gene polymorphisms and bone mineral density (BMD), as a quantitative trait, and fracture risk. There has also been a study suggesting that falls have a hereditary component [15], but despite this, there have been only three studies of which we are aware examining the relationship between gene polymorphisms and falls. A study into vitamin D receptor (VDR) and falls has been previously conducted but in a small group (n = 259) of very elderly (over 80 years) women. This study found an association between Bsm1 and falls, within 90 days of assessment, with a reduction in falls for those having the ‘b’ homozygote, while no association was found for Fok1 [16]. A pharmacogenetic interaction has been noted between PvuII polymorphism of the oestrogen receptor alpha (ESR1) gene and falls [17], where an association was found for women taking hormone replacement therapy (HRT), but there was no association in non-HRT users. In another study examining apolipoprotein E (APOE) polymorphism, no association with falls was found [18]
The focus of research on the role of VDR polymorphisms has been on bone health, and few studies have examined the associations between VDR and muscle strength [19–21, 16]. Two studies [19, 22] have found that the presence of the b allele of the Bsm1 polymorphism is associated with increased quadriceps strength, although other studies have found no such association [20, 21]. Examining lower limb power, the presence of the B allele was associated with increased hamstring strength in one study [20], but no such association was found in another [21]. For grip strength, no significant associations with Bsm1 polymorphism of the VDR gene have been reported [22, 20, 16], although a trend was found for increased grip strength [19] with the ‘b’ allele. If a relationship did exist between VDR gene polymorphisms and falls, this might provide an efficient means of targeting vitamin D treatment to produce an enhanced reduction on fall and fracture rates.
Given the associations found between VDR gene polymorphisms and muscle strength, our aim was to determine the nature of the relationship between VDR gene polymorphisms and fall risk.
Methods
Aberdeen Prospective Osteoporosis Screening Study cohort
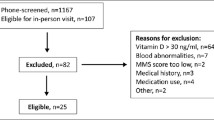
The Aberdeen Prospective Osteoporosis Screening Study (APOSS) population arises from a community-based study, involving women initially aged between 45 and 54 years of age at the baseline visit. The women were chosen at random from a primary care patient register, the Community Health Index, and lived within a 32-km radius of the Osteoporosis Screening Unit, Aberdeen. Between 1990 and 1993 (“baseline”), a letter was sent directly from the screening unit inviting 7,200 potential participants to take part in the screening programme, which consisted primarily of a dual-energy X-ray absorptiometry scan of the hip and spine using a Norland XR-26 and XR-36 Dual-energy X-ray Absorptiometry scanners, and completion of a risk factor questionnaire. Five thousand one hundred nineteen attended for screening.
Between 1997 and 2000 (“visit 2”), the women who had taken part in the study were invited to attend a second screening visit, and 3,883 attended. Falls data were collected using the question “Have you fallen in the last year?” At this time point, those who consented gave a blood sample for DNA analysis. Genotyping of the vitamin D receptor was performed in the Aberdeen bone laboratories using genomic DNA, extracted using standard methodology from peripheral blood leukocytes and stored in 96-well plates at −20°C. Five polymorphic regions of VDR were genotyped. These were the exon 2 Fok1 polymorphism, which creates an alternative translational start site; the functional polymorphism for the Cdx-2 transcription factor binding site, which is located within the promoter region (Cdx-2); and finally, Bsm1, Apa1 and Taq1 in the 3′ region of the gene.
The genotyping methodology has been described in detail previously [23]. Briefly, genotypes for both the ApaI and TaqI polymorphisms were obtained by polymerase chain reaction restriction fragment length polymorphism (RFLP) and visualised on ethidium bromide stained agarose gel. Fok1 and BsmI genotypes were determined primarily (> 89%) using the 5′ nuclease Taqman assay (Applied Biosystems, Foster City, CA, USA) and the remaining samples by RFLP. The concordance rate between the two methods is 98%. The Cdx-2 SNP was genotyped using the DYEnamic ET dye terminator cycle sequencing kit (Amersham Pharmacia Biotech UK) and analysed on a MEGABACE-1000 automated DNA sequencer (Amersham).
Serum samples were analysed for parathyroid hormone (PTH) by a two-site immunoradiometric assay for the intact 84 amino acid chain of PTH (Nichols Institute Diagnostic, San Juan, Capistrano, USA). The assay has been validated by extensive clinical studies in well-defined populations of various parathyroid disease states and is sensitive to 0.9 pM PTH(1–84). The inter-assay coefficient of variation (CV) was 5.6–6.1%, and the intra-assay variation was 1.8–3.4% (measured in samples of mean 40–266 pM). Vitamin D status was determined by measuring 25(OH)D by high-performance liquid chromatography. Serum samples were deproteinised with acetonitrile and centrifuged. The supernatant was introduced onto a Varian Bond Elut C18 cartridge. Roche provided the internal standard. Pre-assayed aliquots of calf serum were used as internal controls. To provide external control, analysts from different laboratories routinely compared results of prepared samples (DEQAS). Within-batch precision was 7.9–13.5% CV, and between-batch precision was 11.9–12.2% CV across the range of 4–100 ng/ml (10–250 nM). Due to the seasonal nature of 25(OH)D [24], we also noted the month the sample was given to allow adjustment for seasonality. Dietary vitamin D assessment was made using a validated food frequency questionnaire and analysed using a UK database [25], and sunlight exposure was assessed using a sunlight questionnaire.
In 2002 (“visit 3”), 2–5 years after the second visit, a postal risk factor questionnaire was sent, and 3,145 returned a completed form. The questionnaire enquired about the presence of incident fractures and falls using the falls question as outlined above for visit 2. Those who indicated the number of falls in the last year (at visit 3 only) to be greater than one were classed as “multiple fallers”.
Those who responded positively to the falls question at both time points (i.e. visits 2 and 3) were classed as “recurrent fallers”.
Written informed consent was obtained from all participants. The Grampian Research Ethics Committee approved the study.
Osteoporosis and Ultrasound cohort
The Osteoporosis and Ultrasound (OPUS) population was recruited in five centres (Aberdeen, Berlin, Kiel, Sheffield and Paris) in 1999 to 2001 (“baseline”). The population has been described in detail elsewhere [26], but in brief, all subjects were randomly selected in age-stratified bands. A younger postmenopausal group of women aged 20 to 40 years was recruited (n = 100 from each centre) but was not included in this analysis. For the older postmenopausal group (aged 55 to 80 years) included in this analysis, each centre aimed to recruit 100 subjects in five 5-year age bands. In total, 2,374 older women were included in this analysis. Serum 25(OH)D was measured in a random sub-set (n = 934) by radioimmunoassay (IDS Ltd, Tyne and Wear, UK). As with the APOSS cohort, we also noted the month the sample was given to allow adjustment for seasonality. Serum PTH (n = 2,269) was analysed using immunoassay (Elecsys 2010 immunoanalyser; Roche Diagnostics GmbH, Mannheim, Germany), with a coefficient of variation of 1.3%. DNA was extracted from whole blood with a modification of the ‘salt-out’ technique (Nucleon II™, Scotlab, Paisley, Scotland). Extracted DNA was suspended at a concentration of 200 μg/ml and stored at −20°C. BsmI and FokI have been genotyped in 1,970 subjects to date using the 5′ nuclease assay (Taqman®; Hoffman-LaRoche, Inc., Nutley, NJ, USA). Falls data were collected using the question “Have you fallen in the last year?” with positive respondents then asked to estimate the number of falls they had suffered.
Balance and muscle power were measured. All subjects had grip strength measured using a dynamometer (Takei Grip-D, Takei Scientific, Japan). Measurements were taken twice in both hands and an average noted of the four measurements. The subjects were also asked to get up from a chair (a chair with arms plus a chair without arms). It was noted by the researcher whether the subject was able to get up “easily”, “with some difficulty”, “with difficulty”, “very difficult” or “not possible”. In addition, subjects were asked to walk along a 5-m line marked on the floor without moving off the line. Each subject was asked to walk on the line forwards with normal steps, backwards with normal steps, forwards toe-to-toe and backwards toe-to-toe. The distance at which they stepped off the line was noted (in 10-cm lengths). A ground reaction force plate was used to measure leg force (n = 578). Each subject was asked to stand up quickly, straightening their knees, and sit down immediately. Three measurements were performed and the average used. Measurements recorded were power (kg), force per weight (N/kg), total force (kN), speed (m/s) and total power (kW).
A second visit was completed between 2005 and 2007 (“visit2”) where further examinations/questionnaires were completed. Written informed consent was obtained from all participants. The study was approved by the appropriate ethics committee at each participating centre.
Multiple fallers are classified as those reporting greater than one fall in the past year (baseline and visit 2). Recurrent fallers are those who indicated at least one fall at both time points.
Statistical analysis
Analyses were performed using SPSS version 15.0 (SPSS Chicago, IL, USA). Logistic regression was used to determine falls risk by calculating odds ratios (OR) and their associated 95% confidence intervals (95% CI). t tests, one-way analysis of variance (ANOVA) and chi-square were also used where appropriate. Chi-square test for Hardy Weinberg equilibrium was performed using Hardy Weinberg Test version 1.10 (Utility Programs for analysis of genetic linkage, J.Ott).
Results
The baseline characteristics of the populations are shown in Table 1. In APOSS, 853 women (22.2%) indicated having sustained a fall in the previous 12 months. In OPUS, where the women are older, 670 of 2,363 (28.4%) indicated a fall at baseline. We categorised the OPUS women according to age group, and the percentage of fallers increased with age (percentage of fallers by age group: 55–59 years = 25%, 60–64 years = 28.2%, 65–69 years = 28.0%, 70–74 years = 28.6% and 75+ years = 33.1%). Using chi-square, this was not statistically significant (χ 2 = 6.17, p = 0.187); however, when a chi-square test for trend was applied, this was statistically significant (χ 2 = 5.05, p = 0.025). Genotype frequencies are shown in Table 2, with chi-square results for deviation from Hardy Weinberg equilibrium. In the Cdx-2 polymorphism, 64.0% of the APOSS cohort was AA homozygote, 31.6% AG heterozygote and 4.4% GG homozygote. In the Fok1, 38.4% of the APOSS cohort was FF homozygote, 46.2% Ff heterozygote and 15.4% ff homozygote, this compared with 34.5% FF homozygote, 49.5% Ff heterozygote and 16.0% ff homozygote in the OPUS cohort. In the Bsm1 polymorphism, 33.9% of the APOSS cohort was bb homozygote, 47.3% Bb heterozygote and 18.9% BB homozygote compared with 35.5% bb homozygote, 49.5% Bb heterozygote and 15.3% BB homozygote in the OPUS cohort. In the Apa1 polymorphism, 31.2% of the APOSS cohort was AA homozygote, 49.8% Aa heterozygote, and 19.0% aa homozygote. In the Taq1 polymorphism, 35.6% of the APOSS cohort was TT homozygotes, 48.2% was Tt heterozygote, and 16.2% was tt homozygote. Table 3 shows the genotype frequencies with regard to faller status. 25(OH)D differed significantly (p = <0.001) when examining the month of measurement for both APOSS and OPUS cohorts.
Vitamin D/PTH and fall risk
APOSS cohort
We compared 25(OH)D and PTH in the fallers and non-fallers at visit 2 only, since the samples were taken at this visit. We found no significant differences in 25(OH)D (p = 0.570), but PTH was significantly higher in the group who had fallen (Mean PTH (SD): fallers = 3.55 ng/L (2.44) vs. non-fallers = 3.32 ng/L (2.05); p = 0.041). It was noted that there was a high SD relative to the mean, suggesting non-normal distribution. However, after ln transformation of these data, the result remained significant (p = 0.012).
OPUS cohort
No significant differences in 25(OH)D were found between those who fell in previous 12 months compared to those who did not fall in OPUS (mean (SD): fallers = 50.1 nmol/L (23.8); non-fallers = 49.8 nmol/L(22.3); p = 0.850). Similarly no significant differences were found comparing multiple fallers vs. fallen once vs. non-fallers for 25(OH)D (mean (SD): multiple fallers = 48.1 nmol/L (20.5); fallen once = 52.3 nmol/L (26.5); non-fallers = 49.8 nmol/L (22.3); p = 0.336). Although we found no significant difference in PTH between fallers and non-fallers (mean (SD): fallers = 38.50 ng/L (21.21); non-fallers = 37.16 ng/L (19.80); p = 0.157), there was a significant difference in PTH for multiple fallers (mean (SD); non-fallers = 37.2 ng/L (19.8); fallen once = 37.0 ng/L (19.0); multiple fallers = 40.4 ng/L (23.5): p value = 0.040).
APOSS cohort
VDR polymorphism and fall risk
With fallers at either time point using the dominant model, BsmI B carriers showed an increase in falls risk, although this was not significant (p = 0.329; Table 4).
None of the other gene polymorphisms showed significant associations with falls at either time point in the APOSS cohort (Cdx-2: p = 0.752; Apa-1: p = 0.525; Taq-1: p = 0.303; Fok-1: p = 0.566; Table 4).
In APOSS, we examined multiple fallers at visit 3 and found an increased risk for carriers of the ‘B’ allele compared to ‘b’ homozygotes (p = 0.047; Table 4). This association remained when adjusting for 25(OH)D and month of measurement (p = 0.043).
None of the other gene polymorphisms showed a significant association with multiple falls at visit 3, although Fok1 did approach significance where ‘f’ homozygote showed an increase in fall risk compared to ‘F’ homozygote (p = 0.054; Table 4).
Recurrent fallers (n = 126), i.e. those reporting a fall at visits 2 and 3, showed an overall trend for increased risk for carriers of the ‘B’ allele when compared to non-fallers (n = 2,398; age, height and weight adjusted OR (95% CI); BB/Bb = 1.527 (1.041–2.298) bb = reference, p = 0.043). This trend remained after adjustment for 25(OH)D and month of measurement (age, height and weight adjusted OR (95% CI); BB/Bb = 1.523 (1.002-2.316) bb = reference, p = 0.049).
None of the other gene polymorphisms showed a significant association with recurrent falls (Cdx-2: p = 0.366; Apa-1: p = 0.401; Taq-1: p = 0.722; Fok-1: p = 0.632).
VDR polymorphisms and vitamin D/parathyroid hormone
Serum 25(OH)D was examined in relationship to VDR polymorphisms. No relationship was found for Cdx2 (p = 0.892), Bsm1 (p = 0.415), Fok1 (p = 0.728), Apa1 (p = 0.956) or Taq1 (p = 0.789). We also examined PTH levels and found only one significant association with Cdx2 (p = 0.023), where those with the ‘G’ homozygote had significantly reduced PTH. No significant associations were found for Bsm1 (0.941), Fok1 (p = 0.937), Apa1 (p = 0.527) or Taq1 (p = 0.776).
OPUS cohort
VDR polymorphism and fall risk
At baseline, we found that carriers of the ‘B’ allele were at significantly increased risks for falls (p = 0.025; Table 4). After further adjustment for 25(OH)D and month of measurement, the association remained significant (p = 0.007).
In the OPUS cohort, grip strength was measured in 658 women allowing additional adjustment. Carriers of the ‘B’ allele were still significantly at increased risk after further adjustment for grip strength (OR (95% CI); BB/Bb = 1.623 (1.112–2.368); bb = reference, p = 0.012).
No significant association was found for Fok1 polymorphism (p = 0.349) and baseline falls.
At baseline, multiple fallers (i.e. those women who reported more than one fall in the previous year), carriers of the ‘B’ allele, showed an even stronger association when adjusted for age, height and weight compared to non-fallers (p = 0.015; Table 4). The significance of the relationship was lost after adjustment for 25(OH)D plus month of measurement (p = 0.083) and, additionally, grip strength (p = 0.104).
No significant associations were found for both Bsm1 and Fok1 with regard to visit 2 falls, visit 2 multiple falls nor with recurrent falls (data not shown).
VDR polymorphisms and vitamin D/parathyroid hormone
In the OPUS cohort, using ANOVA, we compared 25(OH)D levels across the Bsm1 polymorphisms. The bb homozygote was found to have higher levels of 25 (OH)D, although this failed to reach significance (p = 0.09). Similarly, we compared PTH levels across Bsm1 polymorphisms, and this showed a significant difference across genotypes (p = 0.043) with bb showing the lowest PTH level. No differences were found for either 25(OH)D or PTH using the Fok1 polymorphisms (p values = 0.387 and 0.827, respectively).
VDR polymorphisms and balance/muscle power
In the rise from the chair, comparing test subjects who found it “easy” to the remaining subjects, there was a significant difference between the Bsm1 polymorphisms, with more subjects finding it “easy” to rise from a chair with arms (p = 0.023). Similarly, in the rise from a chair without arms, there was a difference between the BsmI polymorphisms, although this failed to reach significance (p = 0.079). Differences between the Bsm1 polymorphisms approached significance for both maximum power (p = 0.080) and the 5-m walk/balance test (forward, normal steps; p = 0.096).
We examined bb homozygotes vs. carriers of the B allele since bb seems to be protective for falls. For the leg force measurements, significant differences were found for maximum power (mean (SD): bb= 0.48 kW (0.15), Bb/BB = 0.45 kW (0.14): p = 0.044), and differences approached significance (p = 0.098) for maximum force (mean (SD): bb = 0.82 kN (0.12), Bb/BB = 0.80 kN (0.13)). There was a difference approaching significance for grip strength (mean (SD); bb = 19.5 kg (5.8), Bb/BB = 18.9 kg (5.6); p = 0.06). None of the 5-m walk/balance test measurements showed significant differences between genotypes (p values from 0.302 to 0.703). For rising from chair, there were significant differences in both rise from chair with arms (% “easy”; bb = 82.3%, Bb/BB = 77.1%; p = 0.008) and rise from chair without arms (% “easy”; bb = 87.4%, Bb/BB = 83.7%; p = 0.030; Table 5).
No associations were found for Fok1 and balance/muscle power measurements.
Vitamin D/PTH and fall risk
No significant differences in 25(OH)D were found between those who fell in previous 12 months compared to those who did not fall in OPUS (mean (SD): fallers = 50.12 nmol/L (23.77); non-fallers = 49.81 nmol/L(22.31); p = 0.850). Similarly, no significant differences were found comparing multiple fallers vs. fallen once vs. non-fallers for 25(OH)D (mean (SD): multiple fallers = 48.08 nmol/L (20.45); fallen once = 52.29 nmol/L (26.50); non-fallers = 49.81 nmol/L (22.31); p = 0.336). Although we found no significant difference in PTH between fallers and non-fallers (mean (SD): fallers = 38.50 ng/L (21.21); non-fallers = 37.16 ng/L (19.80); p = 0.157), there was a significant difference in PTH for multiple fallers (mean (SD); non-fallers = 37.2 ng/L (19.8); fallen once = 37.0 ng/L (19.0); multiple fallers = 40.4 ng/L (23.5): p value = 0.040).
BsmI gene dose effect
Although a monotonic increase was observed in both cohorts, no significant gene dose effect was found in either cohort. In the APOSS cohort, the percentage of women reporting a fall at either time point was 29.8%, 30.0% and 32.3%, respectively, for genotypes bb, Bb and BB (χ 2 p = 0.60; test for trend p = 0.39). Similarly in the OPUS cohort, the percentage of women reporting a fall at either time point was 46.5%, 48.5% and 52.5%, respectively, for bb, Bb and BB (χ 2 p = 0.33; test for trend p = 0.16).
Discussion
Our data show an association between falls and polymorphisms in Bsm1 with carriers of the B allele at higher risk of falls in both cohorts in postmenopausal women. To our knowledge, only one previous study has examined this association [16] between Bsm1 or Fok1 and falls. They found a similar association, with the ‘B’ homozygote showing an increased risk of falls for Bsm1 and no association for Fok1. However, their study was in a very elderly group of women (mean age = 85 years), and the sample size was much smaller than our current study (n = 259). Our current study also shows that the association is independent of serum 25-hydroxy-vitamin D. The effects in our current study were stronger when multiple fallers were compared to non-fallers. The ‘b’ homozygote of Bsm1 was associated with higher serum 25-hydroxy-vitamin D, lower parathyroid hormone and stronger measures of muscle force.
In the APOSS cohort, approximately one fifth reported falling, while this may seem quite high for a relatively young population (mean age 54 years) this is in accordance with fall rates reported elsewhere[27, 28].
In the OPUS cohort, BsmI was associated with falls risk at baseline but was no longer significantly associated at visit2. This may in part be due to loss to follow-up of the frailer members of the cohort. The OPUS cohort are an elderly cohort (mean age 66.9 years). Analysis of a simple general health question revealed that those who did not attend their follow-up visit were more likely to report poorer health, 14.4% versus 10.6% for those who attended.
VDR is a nuclear hormone receptor which was the first to be implicated in osteoporosis [29, 30] when it was noted to relate to BMD. The VDR receptor gene has been targeted in the research of the genetic determinants influencing bone status because it regulates bone homeostasis through the vitamin D endocrine system [31]. The large number of polymorphic sites and a profusion of small and underpowered studies have shown conflicting results for the effects of the polymorphisms on BMD and fractures. Recent examination of all five polymorphisms and their haplotypes in our own population of over 3,000 women failed to show a relationship with BMD or fracture, although with only 248 validated fractures, the latter analysis was underpowered [23]. Indeed, recent studies [32, 33] have shown a relationship between VDR and risk of fracture, which was independent of BMD, but the mechanism by which VDR influences fracture risk was not determined. Also, in a genome-wide search of the top loci associated with BMD, VDR was only found when the search was expanded to the top 1,000 loci [34]. A recent meta-analysis [35] involving over 26,000 participants again showed no significant relationship between any of the five polymorphisms, and BMD but did show a weak relationship between the Cdx-2 polymorphism and vertebral fractures. Assuming this association to be correct, this would indicate an alternative mechanism other than BMD, and one option would be relationships between VDR polymorphisms and falls.
There are a number of limitations associated with this study. Falls data were collected using the question “Have you fallen in the last year?” This has not been validated
In addition, multiple testing has been carried out, although this is justified since the outcomes are correlated. The strength of this study is that this is a large study examining two populations. We have also various measures of muscle power, balance and a measure of 25(OH)D.
Although we found a relationship between these polymorphisms and falls, it is possible that it is a gene located in this area of the chromosome that has yet to be characterised and not the VDR gene that is related to falls. Further work is required to confirm these findings and to elucidate the mechanism of the effect.
References
Kanis JA (2002) Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359:1929–1936
Campbell AJ, Borrie MJ, Spears GF (1989) Risk factors for falls in a community-based prospective study of people 70 years and older. J Gerontol 44:M112–M117
Nevitt MC, Cummings SR, Kidd S, Black D (1989) Risk factors for recurrent nonsyncopal falls. A Prospective Study. JAMA 261:2663–2668
Robbins AS, Rubenstein LZ, Josephson KR, Schulman BL, Osterweil D, Fine G (1989) Predictors of falls among elderly people. Results of two population-based studies. Arch Int Med 149:1628–1633
Lord SR, Sambrook PN, Gilbert C et al (1994) Postural stability, falls and fractures in the elderly: results from the Dubbo Osteoporosis Epidemiology Study. Med J Aust 160:684–685 688-91
Department of Health. How can we help older people not fall again? Implementing the Older People’s NSF Falls Standard: support for commissioning good services. National Service Framework 2003
Cummings SR, Nevitt MC (1989) A hypothesis: the causes of hip fractures. J Gerontol 44:M107–M111
Lord SR, Clark RD, Webster IW (1991) Visual acuity and contrast sensitivity in relation to falls in an elderly population. Age Ageing 20:175–181
Fernie GR, Gryfe CI, Holliday PJ, Llewellyn A (1982) The relationship of postural sway in standing to the incidence of falls in geriatric subjects. Age Ageing 11:11–16
Maki BE, Holliday PJ, Topper AK (1994) A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol 49:M72–M84
Jackson C, Gaugris S, Sen SS, Hosking D (2007) The effect of cholecalciferol (vitamin D3) on the risk of fall and fracture: a meta-analysis. QJM 100:185–192
Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC et al (2004) Effect of vitamin D on falls: a meta-analysis. JAMA 291:1999–2006
Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C (2000) Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res 15:1113–1118
Janssen HC, Samson MM, Verhaar HJ (2002) Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr 75:611–615
Pajala S, Era P, Koskenvuo M, Kaprio J, Viljanen A, Rantanen T (2006) Genetic factors and susceptibility to falls in older women. J Am Geriatr Soc 54:613–618
Onder G, Capoluongo E, Danese P et al (2008) Vitamin D receptor genotype is associated with falls among older adults living in the community: results from the ilSIRENTE study. J Bone Miner Res 23:1031–1036
Salmen T, Heikkinen AM, Mahonen A et al (2002) Relation of estrogen receptor-alpha gene polymorphism and hormone replacement therapy to fall risk and muscle strength in early postmenopausal women. Ann Med 34:64–72
Cauley JA, Zmuda JM, Yaffe K, Kuller LH, Ferrell RE, Wisniewski SR, Cummings SR (1997) Apolipoprotein E polymorphism: a new genetic marker of hip fracture risk—the study of osteoporotic fractures. J Bone Miner Res 14:1175–1181
Geusens P, Vandevyver C, Vanhoof J, Cassiman JJ, Boonen S, Raus J (1997) Quadriceps and grip strength are related to vitamin D receptor genotype in elderly nonobese women. J Bone Miner Res 12:2082–2088
Grundberg E, Brandstrom H, Ribom EL, Ljunggren O, Mallmin H, Kindmark A (2004) Genetic variation in the human vitamin D receptor is associated with muscle strength, fat mass and body weight in Swedish women. Eur J Endocrinol 150:323–328
Windelinckx A, De Mars G, Beunen G et al (2007) (2007) Polymorphisms in the vitamin D receptor gene are associated with muscle strength in men and women. Osteoporos Int 18:1235–1242
Vandevyver C, Vanhoof J, Declerck K et al (1999) Lack of association between estrogen receptor genotypes and bone mineral density, fracture history, or muscle strength in elderly women. J Bone Miner Res 14:1576–1582
Macdonald HM, McGuigan FE, Stewart A et al (2006) (2006) Large-scale population-based study shows no evidence of association between common polymorphism of the VDR gene and BMD in British women. J Bone Miner Res 21:151–162
Macdonald HM, Mavroeidi A, Barr RJ, Black AJ, Fraser WD, Reid DM (2008) Vitamin D status in postmenopausal women living at higher latitudes in the UK in relation to bone health, overweight, sunlight exposure and dietary vitamin D. Bone 42:996–1003
Macdonald HM, New SA, Reid DM (2005) Longitudinal changes in dietary intake in Scottish women around the menopause: changes in dietary pattern result in minor changes in nutrient intake. Public Health Nutr 8:409–416
Gluer CC, Eastell R, Reid DM et al (2004) Association of five quantitative ultrasound devices and bone densitometry with osteoporotic vertebral fractures in a population-based sample: the OPUS Study. J Bone Miner Res 19:782–793
O’Neill TW, Varlow J, Reeve J et al (1995) Fall frequency and incidence of distal forearm fracture in the UK. J Epidemiol Comm Hlth 49:597–598
Luz Rentero M, Carbonell C, Casillas M, Gonzalez Bejar M, Berenguer R (2008) Risk factors for osteoporosis and fractures in postmenopausal women between 50 and 65 years of age in a primary care setting in Spain: a questionnaire. Open Rheumatol J 2:58–63
Morrison NA, Qi JC, Tokita A, Kelly PJ et al (1994) Prediction of bone density from vitamin D receptor alleles. Nature 367:284–287
Kelly PJ, Sambrook PN, Morrison NA, Nguyen T, Eisman JA (1997) Genetics of osteoporosis. World Rev Nutr Diet 80:126–144
Haussler MR, Whitfield GK, Haussler CA et al (1998) The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res 13:325–349
Garnero P, Munoz F, Borel O, Sornay-Rendu E, Delmas PD (2005) Vitamin D receptor gene polymorphisms are associated with the risk of fractures in postmenopausal women, independently of bone mineral density. J Clin Endocrinol Metab 90:4829–4835
Nguyen TV, Esteban LM, White CP et al (2005) Contribution of the collagen I alpha1 and vitamin D receptor genes to the risk of hip fracture in elderly women. J Clin Endocrinol Metab 90:6575–6579
Styrkarsdottir U, Halldorsson BV, Gretarsdottir S et al (2008) Multiple genetic loci for bone mineral density and fractures. N Engl J Med 358:2355–2365
Uitterlinden AG, Ralston SH, Brandi ML et al (2006) The association between common vitamin D receptor gene variations and osteoporosis: a participant-level meta-analysis. Ann Intern Med 145:255–264
Acknowledgements
APOSS study: Thanks go to all the research nurses involved in the APOSS study. Additional thanks for Dr Alison J Black for checking the 25(OH)D and PTH results. Professor William Fraser is thanked for conducting the analysis of the 25(OH)D and PTH samples.
OPUS study: We would like to thank the following members of the OPUS teams at the five participating centres for their contributions: Rosie Reid, Shani Mason, Lindsay Ross, Catherine Paterson, Jennifer Scott and Lana Gibson (Aberdeen); Gabriele Armbrecht, Tilo Blenk, Jessica von der Gablentz, Christina Kahl; Reinhard Barkmann, Wolfram Timm, Christian Graeff and Carsten Rose (Kiel); Sami Kolta (Paris) and Jackie Clowes, Margaret Paggiosi, Nicky Peel and Debbie Swindell (Sheffield). Aubrey Blumsohn measured vitamin D metabolites and PTH in the OPUS study. This project was supported by Sanofi-Aventis, Eli Lilly, Novartis, Pfizer, Proctor and Gamble Pharmaceuticals and Roche.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Rebecca Barr, Helen Macdonald and Alison Stewart contributed equally to the study.
Rights and permissions
About this article
Cite this article
Barr, R., Macdonald, H., Stewart, A. et al. Association between vitamin D receptor gene polymorphisms, falls, balance and muscle power: results from two independent studies (APOSS and OPUS). Osteoporos Int 21, 457–466 (2010). https://doi.org/10.1007/s00198-009-1019-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-009-1019-6