Abstract
The cumulative risk of fracture for a postmenopausal woman over the age of 50 can reach up to 60%. Exercise has the potential to modify fracture risk in postmenopausal women through its effects on bone mass and geometry; however, these effects are not well characterized. To determine the effects of exercise on bone mass and geometry in postmenopausal women, we conducted a systematic review of the literature. We included all randomized controlled trials, cross-sectional studies, and prospective studies that used peripheral quantitative computed tomography to assess the effects of exercise on bone mass and geometry in this population. Exercise effects appear to be modest, site-specific, and preferentially influence cortical rather than trabecular components of bone. Exercise type also plays a role, with the most prominent mass and geometric changes being observed in response to high-impact loading exercise. Exercise appears to positively influence bone mass and geometry in postmenopausal women. However, further research is needed to determine the types and amounts of exercise that are necessary to optimize improvements in bone mass and geometry in postmenopausal women and determine whether or not these improvements are capable of preventing fractures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 30% of postmenopausal women have osteoporosis [1], and the cumulative risk of fracture for a woman in her 50s can reach up to 60% [2, 3]. While there are many pharmacologic agents available for the prevention of osteoporosis, widespread and long-term use of these agents is limited due to side effects [4, 5], costs [6], and poor long-term compliance [7]. Therefore, it is essential that non-pharmacologic strategies to prevent osteoporosis continue to be identified and evaluated.
The value of exercise as a means to prevent postmenopausal bone loss has been explored extensively over the past two decades. Most studies have examined the effects of exercise on bone mineral density (BMD) using dual energy X-ray absorptiometry (DXA). Generally speaking, in postmenopausal women, the effects of exercise on BMD by DXA are site-specific and relatively modest. Aerobics, weight-bearing, and resistance exercises are all effective at preventing some loss of BMD in postmenopausal women, particularly at the lumbar spine; however, these benefits are generally less than 2% [8–10].
While BMD is an important contributor to bone strength, geometric properties (size or shape) of bone also play an essential role. Due to its planar nature, DXA cannot adequately assess geometric properties of bone. In contrast, peripheral quantitative computed tomography (pQCT) can measure bone volume, size, and shape, can distinguish between trabecular and cortical components of bone, and can provide estimations of whole bone strength. Although pQCT cannot measure axial bone sites such as the lumbar spine and hip, which are classic sites for osteoporotic fracture [11], pQCT may be a valuable tool that can provide additional information about the effects of exercise on bone in postmenopausal women that cannot be elucidated by DXA.
To determine the current state of knowledge regarding the effects of exercise on bone mass and geometry assessed by pQCT in postmenopausal women, we conducted a systematic review of the literature.
Methods
An experienced medical librarian conducted a computerized search of three databases (MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials) that included all available published literature until November 2008. The search was conducted using a combination of the following keywords: postmenopausal women, pQCT, physical activity, physical fitness, exercise, exertion, bone geometry, bone structure, and bone architecture. The complete search strategy is available upon request. In addition, two authors (CJH, SAJ) hand-searched references within the published literature on the topic.
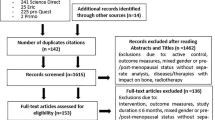
We included all randomized controlled trials, cross-sectional, and prospective studies that used pQCT alone or in addition to other imaging techniques (such as DXA) to assess the effects of exercise training or physical activity participation on bone mass and geometry in postmenopausal women. We did not distinguish between the types of pQCT machines used (Scanco Medical, Zurich, Switzerland versus Stratec Medizintechnik, Pforzheim, Germany), given the limited amount of available literature. Two reviewers (CJH, SAJ) independently examined all potentially relevant abstracts (n = 293) and if there was a disagreement for article inclusion; the paper was reviewed again and a joint decision was made. A summary of the review process is presented in Fig. 1.
For all papers, we reported on bone mass and geometry findings by pQCT as well as by DXA where presented. Whenever possible, we reported the results as mean differences (%) between groups at follow-up. Otherwise, we reported the results in the form in which they were given in the original paper.
Results
Study design, sample size, and statistical methods
Twelve studies met the necessary criteria for inclusion in our review [12–23]; four randomized controlled trials, one non-randomized trial, three cross-sectional studies, and four prospective cohort studies. These studies are summarized in Tables 1 through 3 according to study design.
Sample sizes varied across studies ranging from 98 to 234 participants in the controlled trials, 126 to 239 participants in the cross-sectional studies, and 31 to 208 participants in the prospective investigations.
Statistical methods were valid and comparable across studies. In the controlled trials, general linear modeling techniques were used to report the effects of the exercise interventions on bone outcomes and most studies [16, 17, 20] adjusted for clinically relevant confounders (i.e., age and body weight, etc.). In terms of statistically addressing exercise compliance, two studies [16, 20] provided separate ITT and efficacy analyses and one study adjusted for the average frequency of attendance at the exercise program [12]. Cross-sectional and prospective studies reported on associations between exercise participation and bone outcomes, and for the most part also adjusted for pertinent confounders (all of the cross-sectional studies and two of the prospective studies [15, 21]).
Subject characteristics
Eight of 12 studies assessed postmenopausal women between the ages of 50 and 71 years [12, 13, 18–23]. One study included postmenopausal women under the age of 50 years [14] and three studies examined a more elderly population (women over the age of 72 years) [15–17]. Three studies were in Asian women (two in Hong Kong Chinese women [13, 18], one in Japanese women [14]), six studies were in Finnish women [15, 16, 20–23], one study was in Italian women [12], and two were in North American women (one in American women [19] and one in Canadian women [17]). Years since menopause (YSM) ranged from less than 10 years in five studies [13, 14, 18–20] to 10 and 20 years in four studies [12, 21–23], and greater than 29 years in one study [17]. Two studies did not report YSM as all participants were over the age of 70 years [15, 16]. Mean body mass index was <30 kg/m2 in all studies. Exclusion criteria was similar in most studies and generally excluded individuals who were chronically ill, had a prior history of fracture, were currently using hormone therapy or medications known to affect bone metabolism, or had conditions known to affect bone metabolism such as hypo- and hyperthyroidism, hypo- and hyperparathyroidism, or renal and liver disease. Two studies [17, 23] included women on bisphosphonates or estrogen therapy; however, the use of these medications was adjusted for in the analyses.
Exercise assessment
The exercise programs used in the controlled trials varied in frequency, duration, training period, and type of exercise. The frequency of training ranged from two to five times per week, the session durations lasted anywhere between 50 and 100 min, and the training periods were either 6 or 12 months in length. Several types of exercise were assessed, including: high-impact bone loading activities (volleyball, multidirectional jumping), low-impact weight-bearing Tai Chi Chuan, resistance training, balance and agility exercises, or a combination of exercise types. The majority of training programs targeted the lower limbs. All studies recruited subjects who had no prior history of regular exercise and were not currently participating in any form of exercise on a consistent basis. Generally speaking, control subjects were counseled to maintain their current level of physical activity throughout the trial, with the exception of one trial where control subjects participated in sham exercise (stretching) [17]. Mean training compliance across trials was generally 70% or better.
Cross-sectional and prospective studies assessed participation in leisure physical activity as well as specific sports, including: resistance and balance-jumping training, Finnish gymnastics, volleyball, and Tai Chi Chuan. The length of prospective investigations ranged anywhere from 1 to 6 years.
Bone mass and geometry assessment
In all studies, bone mass and geometry measures were made using low-resolution pQCT technology. Nine studies used single slice Stratec machines: XCT 540 (one study), XCT 960 (one study), and XCT 3000 (seven studies); and three studies used multislice Scanco machines: Densiscan 1000 (one study) and Densiscan 2000 (two studies). None of the studies used high-resolution pQCT (HR-pQCT) which makes the included studies comparable in terms of image resolution and output. Six studies assessed the tibia [13, 14, 18, 20, 21, 23], one study assessed the radius [12], four studies assessed both the radius and the tibia [15–17, 22], and one study assessed the radius and femur [19]. In all studies, both proximal and distal bone sites were assessed.
Bone mass and geometry variables reported on at distal sites included: total bone mineral content (BMC), total area, trabecular area, total BMD, cortical BMD, cortical organ density, trabecular BMD, integral BMD, density-weighted polar section modulus, strength–strain index, ratio of cortical to total area of bone, periosteal circumference, and polar moment of inertia. At the proximal sites, total BMC, total cross-sectional area, medullary cavity area, cortical BMC, cortical area, cortical thickness, cortical BMD, periosteal circumference, endosteal circumference, density-weighted polar section modulus, strength–strain index, and polar moment of inertia were assessed. For simplicity and ease of comparison among studies, in this manuscript, we will collectively refer to the terms moment of inertia, density-weighted section modulus, bone strength index, and strength–strain index (all of which estimate a bone’s bending and torsional strength) as bone strength (BS).
DXA and pQCT findings
Controlled trials
Four randomized controlled trials and one non-randomized trial reported site-specific benefits of various types of exercise training programs on bone mass and geometry in postmenopausal women (Table 1). Adami et al. [12] evaluated the effects of a 6-month training program designed to load the wrist (volleyball, impact exercises), on bone mass, and geometry at the radius in a group of postmenopausal women (n = 234). DXA was used to assess bone mass at the lumbar spine, hip, and radius. pQCT was used to assess bone mass as well as geometry at the radius. DXA measurements at the hip, spine, and radius were similar between groups before and after intervention. The lack of training effects observed at the hip and spine sites were not surprising given that the training program was designed to specifically load the wrist, and that training effects are typically site-specific [24]. However, the discordant DXA and pQCT findings at the radius were noteworthy. While DXA measures were unremarkable post training at the radius, pQCT measures revealed that exercisers had greater cortical area, cortical BMC, and smaller trabecular BMD at the ultradistal radius than controls, structural modifications that are associated with improved bone strength.
Uusi-Rasi et al. [20] reported similar findings in a study that examined the effects of oral alendronate and weight-bearing jumping exercises on bone mass and geometry at the hip, spine, and lower leg in postmenopausal women (n = 152). Women in this study were randomly assigned to one of four experimental groups over 12 months: (1) 5 mg of alendronate daily plus exercise, (2) 5 mg of alendronate, (3) placebo plus exercise, or (4) placebo. Bone mass at the lumbar spine, hip, and radius were assessed by DXA and pQCT was used to examine bone mass and geometry at the tibia. Exercise alone had no effect on bone mass at the lumbar spine, hip, or radius. Moreover, it had neither an additive nor an interactive effect with alendronate. However, post training, exercisers had a greater ratio of cortical bone to total bone at the distal tibia compared to controls. BS and cortical area were also greater among exercisers at the distal tibia and tibial shaft, respectively. Similar to the findings of Adami et al. [12], site-specific impact exercises seemed to re-shape cortical components of bone, thereby improving bone strength.
Karinkanta et al. [16] also reported improvements in BS among postmenopausal women randomized to a 12-month resistance and balance-jumping exercise program targeting the lower limbs. Women in this study (n = 144) were randomly assigned to three different types of training: (1) resistance training, (2) balance-jumping training, or (3) a combination of resistance and balance-jumping training. DXA was used to assess bone mass and geometry at the hip and pQCT was used to assess bone mass and geometry at the radius and tibia. The combination of resistance and balance-jumping training was effective at maintaining BS at the tibial shaft among exercisers (BS decreased 2% less) compared with controls, but did not affect bone mass or geometry at any other sites. The resistance and balance-jumping training programs alone did not affect bone mass or geometry at any measured sites.
Liu-Ambrose et al. [17] examined the effects of resistance and agility training on bone at the radius and tibia in elderly women with (n = 98) with low BMD (osteopenic/osteoporotic). Women were randomized to three training groups: (1) resistance training (targeting upper limbs, lower limbs, and trunk), (2) agility training (targeting lower limbs), or (3) stretching (control). DXA was used to assess bone mass at the hip and pQCT was used to assess bone mass and geometry at the radius and tibia. Post training, cortical BMD was greater in the resistance training group compared with the agility training group at the radial shaft and greater in the agility training group compared with the control group at the tibial shaft. However, unlike the aforementioned studies, no significant between-group differences were observed for bone geometry or strength measures.
A final study by Chan et al. [13] evaluated the benefits of Tai Chi Chuan (TCC) exercise on the tibia in postmenopausal women (n = 103) randomized to either TCC exercise or no exercise over 12 months. Bone mass was assessed at the lumbar spine and hip using DXA and at the tibia using pQCT. Post intervention, bone loss was observed in both TCC and control groups. However, BMD loss among exercisers was significantly slower in both trabecular and cortical compartments of the distal tibia compared to controls. These findings suggest that even low-impact exercise like TCC may help to maintain volumetric BMD at loaded bone sites in postmenopausal women.
Cross-sectional studies
All three cross-sectional studies reported positive associations between physical activity participation and bone mass and geometry in postmenopausal women (Table 2). Uusi-Rasi et al. [23] evaluated the benefits of long-term participation in Finnish recreational gymnastics on bone mass and geometry at the hip, radius and tibia, by comparing healthy postmenopausal women regularly participating in gymnastics (n = 117) with non-gymnasts (n = 116). In this study, DXA was used to assess bone mass at the hip and radius, and pQCT was used to assess bone mass and geometry at the tibia. No differences in BMC or BMD were observed between gymnasts and non-gymnasts at the hip or radius. However, gymnasts had greater BMC, total area and trabecular BMD at the distal tibia than the sedentary women. Gymnasts also had greater BMC and cortical area at the tibial shaft. The lack of significant findings observed between groups at the hip and radius is consistent with the existing knowledge that training effects are typically site-specific and generally only affect loaded bone sites [24]. In this case, Finnish recreational gymnastics primarily loads the lower limbs, and therefore benefited both bone mass and geometry of both trabecular and cortical bone.
Another study by Uusi-Rasi et al. [22] examined the impact of habitual physical activity (PA) participation and calcium intake on bone geometry at the radius and tibia in healthy women, 126 of them were postmenopausal. Based on the results of a questionnaire, women were divided into groups according to their habitual levels of PA (PA+ or PA−) and calcium intake (Ca+ or Ca−). Bone mass and geometry variables assessed by pQCT at the radius and tibia were then compared among groups. Postmenopausal women in the physically active group (PA+) had greater BS at the radial shaft than inactive women (PA−), suggesting a mechanically more competent radial cortical structure among the physically active women. No differences were found between PA+ and PA− groups at the distal radial site. At the tibia, the PA+ group had greater BMC at the distal site, as well as greater BMC, cortical area and overall BS at the shaft site than the PA− group. The multiple associations observed at radial and tibial shaft sites, suggest that cortical bone components may be more sensitive to loading effects than trabecular components. Further, a more active lifestyle may be beneficial for bone strength.
A final study by Shedd et al. [19] contradicts data from the aforementioned studies by reporting both positive as well as negative associations between physical activity and bone mass and geometry in postmenopausal women. This study compared three methods of quantifying physical activity [peak strain score (PSS), hip bone loading exposure score (BLE) and total activity score (TAS)] to identify which method had stronger associations with BMD at the hip, spine, and femoral neck assessed by DXA, as well as with volumetric density and strength measures at the distal tibia and midshaft femur assessed by pQCT. Postmenopausal women (n = 239) from Iowa (ISU) and California (UCD) completed the Paffenberger physical activity questionnaire, which was scored with each of the three methods. No method was associated with BMD by DXA at any bone site. At the tibia, PSS was negatively associated with measures of BS, but no significant associations were found with the TAS or hip BLE scores. At the femur, TAS was positively associated with cortical area and cortical thickness. The positive findings from this study are in line with the aforementioned data which suggests that physical activity can benefit cortical bone at load-bearing sites such as the femur. The negative associations are surprising and contradict the majority of literature that indicates that exercise has a beneficial effect on bone [25]. However, the authors state that, because the UCD women were more active than ISU women, one group may have negated the effects of the other when pooled, which could have accounted for the negative findings.
Prospective studies
Three of four prospective studies reported longitudinal benefits of exercise participation on bone mass and geometry in postmenopausal women (Table 3). Ito et al. [14] evaluated the effects of habitual volleyball practice on bone mass at the lumbar spine and calcaneus (by DXA) and bone mass and geometry at the tibial diaphysis (by pQCT) in a group of pre-, peri-, and postmenopausal women. Women belonging to a non-professional volleyball club for more than 10 years were compared with sedentary women. At baseline, postmenopausal volleyball players (n = 20) had greater areal BMD at the lumbar spine and calcaneus compared to sedentary women (n = 35). Volleyball players also had greater volumetric BMD, cortical area, and BS at the tibial diaphysis. Annual rates of BMD loss (over 12 and 24 months) at the calcaneus and tibial diaphysis were slower in volleyball players than sedentary women, suggesting a potential role for recreational volleyball in the prevention of age-related areal and volumetric BMD loss at the calcaneus and tibia, sites that are heavily loaded by volleyball.
A similar study by Qin et al. [18] compared bone mass and geometry in TCC exercisers with sedentary women. Bone mass was assessed at the lumbar spine and hip by DXA and bone mass and geometry were assessed at the tibia by pQCT. At baseline, regular TCC exercisers (DXA n = 15, pQCT n = 16) had greater areal BMD at the lumbar spine and hip than sedentary women (DXA n = 11, pQCT n = 15). TCC exercisers also had greater trabecular BMD at the ultradistal tibia. At 12-month follow-up, no differences were observed between groups for areal BMD measures at the hip and spine; however, TCC exercisers had slower cortical BMD loss at the distal tibial diaphysis and slower trabecular BMD loss at the ultradistal tibia compared with the sedentary women, suggesting a role for TCC in the maintenance of both trabecular and cortical BMD in postmenopausal women.
Longitudinal benefits of exercise participation were also demonstrated in a 6-year prospective study by Uusi-Rasi et al. [21] (follow-up to a previous cross-sectional study Uusi-Rasi et al. 1999 [23]; see Table 2) evaluating the effects of long-term participation in recreational Finnish gymnastics on bone mass and geometry at the hip (by DXA) and tibia (by pQCT) in postmenopausal women (n = 208). Bone mass and structural strength decreased at a similar rate in both gymnasts and sedentary referents over the 6-year period. However, compared with sedentary referents, gymnasts had consistently higher mean bone values at measured bone sites, indicating that many of benefits reported in the previous study had been preserved.
In a final study by Karinkanta et al. [15], postmenopausal women who participated in a previous randomized controlled trial (n = 121) were followed up 12 months after cessation of the intervention to assess the maintenance of the original exercise effects of a resistance and balance-jumping training program (see Karinkanta et al. 2007 [16]; Table 1). In the original randomized controlled trial, the combination training group had greater BS at the tibial shaft compared to the control group. At 1-year follow-up post intervention, this effect was no longer present, suggesting that the maintenance of loading effects on the skeleton may depend on continual exercise exposure.
Discussion
Summary of findings
Collectively, this review suggests that exercise is capable of modifying bone mass and geometry in postmenopausal women, adaptations that may theoretically improve bone strength. Specifically, training effects appear to be modest, site-specific, and primarily affect cortical rather than trabecular components of bone.
The trend for exercise to primarily influence cortical components of bone prevailed in both interventional as well as observational studies. Most studies reported benefits of training on cortical BMD, cortical area, and/or bone strength at both distal and shaft sites of loaded bone segments [12–14, 16–20, 22, 23]. The effects of exercise on trabecular components of bone were less definitive. Of the eight studies that assessed trabecular bone measures, only four [12, 13, 18, 23] showed changes in response to exercise. Generally, exercise helped to maintain trabecular BMD at distal sites of loaded bone segments; however, results were heterogeneous, with one study showing a loss of trabecular BMC with training [12].
The ability of exercise to primarily affect cortical components of bone has important clinical implications. First, bones increase in size as we age [26–29], an adaptation that enhances structural competence, as a larger cross-sectional area and cortical diameter allows for better resistance to bending and compressive loads [30]. Exercise seems to enhance this age-related adaptation, which may in turn decrease fracture risk. Second, it is plausible that exercise may counteract natural age-related changes in bone structure observed in postmenopausal women. Recent reports suggest that bone fragility in postmenopausal women appears to be a consequence of both reduced periosteal bone formation and increased endocortical resorption [31, 32]. As exercise has been shown to improve both cortical area and cortical BMD, as well as slow trabecular bone loss, it may play a role in mediating these effects. Finally, the effects of exercise on cortical bone may also be particularly complimentary to current drug therapies for osteoporosis which are primarily known to have greater affects on trabecular components of bone [33].
Our review also highlighted that a variety of exercise types can be beneficial for bone geometry and the type of exercise seems to play a role in influencing the degree of the training effect. In general, physical activities previously reported to have the most substantial effect on the skeleton include those that involve: (a) high-impact, rapid, forceful loading (e.g., running, jumping, gymnastics, volleyball); (b) changing, diverse, or novel loading angles (e.g., ball sports, gymnastics); (c) weight-bearing, high forces (e.g., dancing, weight lifting); and (d) a direct impact on the bone of interest (e.g., dominant arm of tennis players) [34]. For the most part, our review was consistent with these findings. In the controlled trials, the most substantial changes in bone mass and geometry were reported in response to high-impact loading activities like volleyball and jumping [12, 20], more moderate effects were reported with resistance and agility training [17], and the smallest effects were observed with low-impact activities like TCC [13]. However, in the study by Karinkanta et al. [16], exercise effects were achieved only when all three aforementioned training types were combined.
Limitations
The studies included in our review had several limitations. Of the 12 published studies, only four were randomized trials. The absence of randomization in more than half of the available literature introduces a potential for bias. Further, sample sizes were small in the majority of studies, and there was a large variation in the types of exercise assessed. In addition, the lack of standardization among pQCT scanning techniques as well as the diversity of variables examined and skeletal sites at which bone geometry was assessed makes it difficult to compare and synthesize findings.
The majority of studies were in Asian (Chinese and Japanese) and European (Finnish and Italian) women. The prevalence of osteopenia and osteoporosis as well as patterns of postmenopausal bone loss varies in a predictable manner with respect to race and ethnicity [35]. Therefore, results from the studies to date cannot be generalized to all populations of women. Further, women included in these studies had baseline BMD scores ranging from normative to osteoporotic. The responsiveness of BMD to exercise may vary with the degree of BMD loss severity, and thus may influence exercise effects. The age range of women included in these studies was also large. As rates of bone loss vary with age [36, 37], comparison of exercise effects between study groups is difficult. Based on findings from this review and from the current literature [38], there is evidence to suggest that younger women may reap more skeletal benefits from exercise. One reason for this may be the fact that exercise intensity is difficult to maintain with ageing [21], as ageing is associated with muscle atrophy and with degenerative changes in peripheral nerve and neuromuscular junctions that result in muscle weakness [39]. As muscles exert large forces on bones, decreased muscle strength may inhibit older individuals from maintaining exercise intensity at a sufficient level to cause changes in bone. Exercise effects were lessened in the two interventional studies assessing older populations of women [16, 17], which is consistent with this hypothesis.
Finally, there is limited data on the effects of leisure physical activity on bone mass and geometry in postmenopausal women. The exercise training programs employed in the interventional trials were specialized, and may not be representative of programs that can be adhered to in a non-interventional setting, or that are attainable for populations of women that are more frail and limited in their ability to perform vigorous activity. Physical activity that is community based and accessible to populations of women that cannot participate in institutionally based activity programs needs to be explored.
Conclusions
This review highlights the importance of examining bone mass and geometry by pQCT in addition to DXA when assessing the skeletal response to exercise training in postmenopausal women. In almost all of the studies examined, changes in bone mass and geometry were evident by pQCT even when DXA measurements were unremarkable. Although most of the measured bone sites with pQCT and DXA differed, pQCT measures provided additional information about the effects of exercise on bone that could not be elucidated by DXA. Therefore, using pQCT as well as DXA may provide a more complete picture of the effects of exercise on bone in postmenopausal women.
In summary, exercise appears to positively influence bone mass and geometry in postmenopausal women, but effects are modest and appear to be dependent on continued participation in exercise as well as on the ability to maintain sufficient exercise intensity. More research is necessary to fully understand what types of exercise and how much exercise is best to achieve improvements in bone mass and geometry, and whether such adaptations are in fact capable of preventing fractures.
References
Kanis JA (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 4:368–381
Cooper C (1993) The epidemiology of fragility fractures: is there a role for bone quality? Calcif Tissue Int 53(Suppl 1):23–26
Cummings SR, Black DM, Rubin SM (1989) Lifetime risks of hip, Colles’, or vertebral fracture and coronary heart disease among white postmenopausal women. Arch Intern Med 149:2445–2448
Cryer B, Bauer DC (2002) Oral bisphosphonates and upper gastrointestinal tract problems: what is the evidence? Mayo Clin Proc 77:1031–1043
Rossouw JE, Anderson GL, Prentice RL et al (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333
Wiktorowicz ME, Goeree R, Papaioannou A et al (2001) Economic implications of hip fracture: health service use, institutional care and cost in Canada. Osteoporos Int 12:271–278
Weycker D, Marcarios D, Edelsberg J et al (2006) Compliance with drug therapy for postmenopausal osteoporosis. Osteoporos Int 17:1645–1652
Bonaiuti D, Shea B, Iovine R et al (2002) Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev 3:CD000333
Shea B, Bonaiuti D, Iovine R et al (2004) Cochrane Review on exercise for preventing and treating osteoporosis in postmenopausal women. Eura Medicophys 40:199–209
Wallace BA, Cumming RG (2000) Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif Tissue Int 67:10–18
Johnell O, Kanis J (2005) Epidemiology of osteoporotic fractures. Osteoporos Int 16(Suppl 2):S3–S7
Adami S, Gatti D, Braga V et al (1999) Site-specific effects of strength training on bone structure and geometry of ultradistal radius in postmenopausal women. J Bone Miner Res 14:120–124
Chan K, Qin L, Lau M et al (2004) A randomized, prospective study of the effects of Tai Chi Chun exercise on bone mineral density in postmenopausal women. Arch Phys Med Rehabil 85:717–722
Ito M, Nakamura T, Ikeda S et al (2001) Effects of lifetime volleyball exercise on bone mineral densities in lumbar spine, calcaneus and tibia for pre-, peri- and postmenopausal women. Osteoporos Int 12:104–111
Karinkanta S, Heinonen A, Sievanen H et al (2008) Maintenance of exercise-induced benefits in physical functioning and bone among elderly women. Osteoporos Int . doi:10.1007/s00198-008-0703-2
Karinkanta S, Heinonen A, Sievanen H et al (2007) A multi-component exercise regimen to prevent functional decline and bone fragility in home-dwelling elderly women: randomized, controlled trial. Osteoporos Int 18:453–462
Liu-Ambrose TY, Khan KM, Eng JJ et al (2004) Both resistance and agility training increase cortical bone density in 75- to 85-year-old women with low bone mass: a 6-month randomized controlled trial. J Clin Densitom 7:390–398
Qin L, Au S, Choy W et al (2002) Regular Tai Chi Chuan exercise may retard bone loss in postmenopausal women: a case–control study. Arch Phys Med Rehabil 83:1355–1359
Shedd KM, Hanson KB, Alekel DL et al (2007) Quantifying leisure physical activity and its relation to bone density and strength. Med Sci Sports Exerc 39:2189–2198
Uusi-Rasi K, Kannus P, Cheng S et al (2003) Effect of alendronate and exercise on bone and physical performance of postmenopausal women: a randomized controlled trial. Bone 33:132–143
Uusi-Rasi K, Sievanen H, Heinonen A et al (2006) Long-term recreational gymnastics provides a clear benefit in age-related functional decline and bone loss. A prospective 6-year study. Osteoporos Int 17:1154–1164
Uusi-Rasi K, Sievanen H, Pasanen M et al (2002) Associations of calcium intake and physical activity with bone density and size in premenopausal and postmenopausal women: a peripheral quantitative computed tomography study. J Bone Miner Res 17:544–552
Uusi-Rasi K, Sievanen H, Vuori I et al (1999) Long-term recreational gymnastics, estrogen use, and selected risk factors for osteoporotic fractures. J Bone Miner Res 14:1231–1238
Kannus P, Haapasalo H, Sankelo M et al (1995) Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med 123:27–31
Kohrt W, Snead D, Slatopolsky E et al (1995) Additive effects of weight-bearing exercise and estrogen on bone mineral density in older women. J Bone Miner Res 10:1303–1311
Atkinson PJ, Weatherell JA (1967) Variation in the density of the femoral diaphysis with age. J Bone Joint Surg Br 49:781–788
Ruegsegger P, Durand EP, Dambacher MA (1991) Differential effects of aging and disease on trabecular and compact bone density of the radius. Bone 12:99–105
Smith RW Jr, Walker RR (1964) femoral expansion in aging women: implications for osteoporosis and fractures. Science 145:156–157
Wapniarz M, Lehmann R, Reincke M et al (1997) Determinants of radial bone density as measured by PQCT in pre- and postmenopausal women: the role of bone size. J Bone Miner Res 12:248–254
Martin RB (1991) Determinants of the mechanical properties of bones. J Biomech 24(Suppl 1):79–88
Lauretani F, Bandinelli S, Griswold ME et al (2008) Longitudinal changes in BMD and bone geometry in a population-based study. J Bone Miner Res 23:400–408
Szulc P, Seeman E, Duboeuf F et al (2006) Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res 21:1856–1863
Silcox D (2008) Updates on bisphosphonates for treatment of osteoporosis. Available via: http://www.opocenter.com/updates/update3.html Accessed 15 Oct 2008
Singh M (2004) Exercise and bone health. In: Holick M, Dawson-Hughes B (eds) Nutrition and bone health. Humana, Totowa, pp 515–548
U.S. Department of Health and Human Services (2004) Bone health and osteoporosis: a report of the Surgeon General. Office of the Surgeon General. Available via: http://www.surgeongeneral.gov/library/bonehealth/content.html Accessed 3 Nov 2008
Ensrud KE, Palermo L, Black DM et al (1995) Hip and calcaneal bone loss increase with advancing age: longitudinal results from the study of osteoporotic fractures. J Bone Miner Res 10:1778–1787
Nilas L, Christiansen C (1988) Rates of bone loss in normal women: evidence of accelerated trabecular bone loss after the menopause. Eur J Clin Invest 18:529–534
Bassey EJ, Rothwell MC, Littlewood JJ et al (1998) Pre- and postmenopausal women have different bone mineral density responses to the same high-impact exercise. J Bone Miner Res 13:1805–1813
Rolland Y, Czerwinski S, Abellan Van Kan G et al (2008) Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging 12:433–450
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamilton, C.J., Swan, V.J.D. & Jamal, S.A. The effects of exercise and physical activity participation on bone mass and geometry in postmenopausal women: a systematic review of pQCT studies. Osteoporos Int 21, 11–23 (2010). https://doi.org/10.1007/s00198-009-0967-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-009-0967-1