Abstract
Summary
Telomere length (TL), as a reflection of aging and inflammatory processes, may be associated with bone mineral density (BMD). This study examines the association between TL and BMD cross-sectionally and the rate of bone loss over a 4-year period in 1,867 Chinese elderly community living subjects. After adjusting for confounding factors, no association was observed with BMD or bone loss. The decline in BMD with aging is not reflected by corresponding changes in telomere length.
Introduction
Bone mineral density (BMD) is influenced by the dynamics of aging, inflammatory, and bone remodeling processes. Telomere length (TL) is a reflection of the former two processes and may also be associated with bone loss.
Methods
Hip BMD was measured in 1,867 Chinese elderly community living subjects and the relationship between leukocyte TL measured using quantitative real-time polymerase chain reaction, and bone loss after 4 years was examined.
Results
Women had greater bone loss than men. In women, age of menopause, menarche, estrogen treatment/replacement therapy, and history of previous fracture were also among the significant covariates. However, in multivariate analyses, TL was not associated with BMD in either sex.
Conclusions
TL was not associated with either baseline BMD or bone loss over 4 years and accounted for less than 1.6% of the baseline BMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis represents a major public health concern in aging societies, fragility fractures having the highest impact on both quality of life and health care systems worldwide [1]. Bone mineral density measurement (BMD) in hip and spine is used in the diagnosis of osteoporosis [2]. The association between low BMD and risk of bone fractures has been reported extensively [3–7]. In a recent review, advanced age, smoking, low body weight, physical limitations, and prevalent fracture (after age 50) were consistent risk factors for osteoporosis [8]. BMD declines with age, chronological age being a major determinant of BMD.
Recent studies into the mechanism of aging revealed various processes leading to replicative senescence [9]. Critically short telomere length is a marker of biological aging [10]. In addition to natural replicative attrition, the inflammatory process also enhances the rate of telomere length (TL) attrition, such that association between TL and chronic inflammatory states has been documented. Telomeres consist of short unique repeated DNA sequence (TTAGGG), and specialized proteins are located at both ends of linear chromosomes in humans. They act as the cap of the chromosome and maintain genomic integrity by preventing chromosome fusions and atypical recombination [11]. Due to the end replication problem of DNA, telomeres shorten in each cell division. Therefore, telomere length and its attrition reflect on cellular replication history and cellular senescence and is considered a new marker of biological aging, representing a new but different dimension of the aging process from chronological age. With respect to bone health, replicative aging of osteoblast precursor cells has been demonstrated and shown to be associated with telomere attrition in this cell type [12]. Furthermore, exacerbated telomere erosion is also a common feature in other diseases associated with chronic inflammation such as autoimmune and ischemic heart diseases [13, 14].
There are genetic and environmental determinants for BMD. Genetic determinants of BMD have been described extensively [15–18]. Genetic studies include candidate gene association, familial linkage, and genome-wide association (GWA) studies [19, 20]. On the other hand, smoking, immobility, and chronic inflammatory conditions have been documented as common environmental risk factors associated with osteoporosis. It is interesting to find that pro-inflammatory genes were also associated with osteoporosis in the latest GWA studies. For example, one of the predisposition genes for osteoporosis is the receptor activator of nuclear factor κB ligand (RANKL) [21]. Chronic inflammation with activated T cells induced the expression of RANKL gene and enhanced bone resorption. These results indicate a strong role of inflammatory response in bone loss [22–24].
Since both bone loss and telomere attrition have been linked to inflammation, it has been hypothesized that short telomere length is a risk factor for osteoporosis. In a small cohort of 84 elderly men, telomere length was not associated with baseline forearm BMD, but it was associated with bone loss at a borderline significance (P = 0.03) [25]. In another study of 2,150 women aged between 18 and 80, telomere length was significantly correlated with forearm and spine BMD [26]. These findings suggest that telomere length may be a predictor or marker for osteoporosis and impending bone loss in aging populations. To date, there have not been any studies in Chinese populations. As part of a territory-wide study on bone health in older people aged 65 and over, we examined the association between telomere length, baseline BMD, and its change over 4 years.
Methods
Study subjects
A community cohort of 2,000 men and 2,000 women aged 65 or above were recruited from community centers for the elderly and housing estates in Hong Kong. In the recruitment process, about one third of the volunteers were stratified to each of the age groups of 65–69, 70–74, and 75 or above. DNA samples were available and analyzed in a subgroup of 963 men and 904 women. At recruitment, participants completed a detailed questionnaire containing information on demographics, medical and drug histories, smoking status, and dietary intake [27]. The presence or absence of diseases was reported as diagnosed by their doctors. The study had been approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong, and informed consent was obtained from all participants.
Bone mineral density at the hip and femoral neck was measured using dual X-ray absorbtiometry (DXA) by Hologic QDR-4,500 W densitometers (Hologic, Waltham, MA, USA). The coefficient of variation (in vivo) in our laboratory was 0.7% at the hip [28]. Clinical osteoporosis at the hip was defined using the WHO criteria of a T-score less than −2.5. Body weight was measured with subjects wearing a light gown using the Physician Balance Beam Scale (Healthometer, Illinois, USA). Height was measured by the Holtain Harpenden standiometer (Holtain, Crosswell, UK). Body mass index (BMI) was calculated by dividing the weight in kilograms by the square of the height in meters.
Information about usual level of physical activity was also recorded using the Physical Activity Scale of the Elderly (PASE). This is a 12-item scale measuring the average number of hours per day spent in leisure, household, and occupational physical activities over the previous 7-day period. Activity weights for each item were determined based on the amount of energy expended, and each item score was calculated by multiplying the activity weight by activity daily frequency. A summary score of all the items reflect the daily physical activity level [29].
Four-year follow-up
All participants were invited for a 4-year follow up. During the follow-up examination, information on disease status and fracture history was recorded. Anthropometry and BMD were also measured. Changes in BMD were determined during the 4-year period. Seven hundred twenty-seven men and 735 women came back for follow-up.
Telomere length measurement by quantitative real-time polymerase chain reaction
The principle of the laboratory method has been previously described [27]. In short, DNA was extracted in the peripheral blood by phenol-chloroform method and stored at −80°C with concentration >100 ng/μl (whereas working samples of 20 ng/μl were prepared before analysis). Telomere length measurement followed the method published by Cawthon [30] with modification [31]. Unlike the traditional method using terminal restriction fragment (TRF) analysis, this method used the technique of quantitative real-time polymerase chain reaction (qRT-PCR), which has the advantage of high throughput, time-saving, and reproducible estimation of telomere length [30, 32–34]. Roche LightCycler 480 (Roche, Mannheim, Germany) was used to perform the qRT-PCR with primer sequences obtained from Cawthon [31]. The T/S ratio (Ct(telomere assay)/Ct(single-copy gene assay)) was used to assess the relative length of telomere, while C t is the fractional cycle number for a threshold fluorescence level to be reached during qRT-PCR. The T/S ratio (ΔΔCt) was then plotted against a standard calibration curve using samples with predetermined telomere lengths to obtain the telomere length in unit of kilobase pairs (kbp). The coefficient of variation % (CV%) of the Ct measurements of the telomere and the single-copy gene probes were 3.6% and 1.8%, respectively. Two control samples were analyzed in duplicates in each batch of assays; one sample was collected from volunteers of age of 20s representing a long telomere control (long QC, by TRF = 11.6 kbp) and another one from an elderly subject representing a short telomere control (short QC, by TRF = 8.2 kbp). Within-batch and between-batch analytical imprecisions were determined from over 20 batches of assays using these two samples. The within-batch and between-batch CV% of ΔΔCt was 11.9% and 11.2% for the long QC sample. For the short QC sample, they were 8.1% and 14.2%, respectively.
A calibration curve was drawn by using ΔΔCt values obtained from five different samples of predetermined TL by TRF method. The coefficient of determination (R 2) of the linear correlation between TL by TRF method and ΔΔCt was 0.63, which was similar to that reported (R 2 = 0.68) in the original description of the ΔΔCt method by Cawthon [22]. Calibration to TL in kbp was carried out independently for each batch which in a way partially corrected for between-batch variation in determination of ΔΔCt and TL. After calibration to TL in kbp, the within-batch and between-batch CV% of TL was 8.5% and 7.5% for the long QC sample. For the short QC sample, they were 6.3% and 6.1%, respectively. A reduction of between-batch CV% was noted which reflected a partial correction of the between-batch variation in the calibration process.
Statistical analysis
Descriptive and analytical statistics were carried out using SPSS v15.0 (SPSS, Chicago, USA). Analytical errors of ΔΔCt and TL were partitioned into within-batch and between-batch errors using a BASIC program [35]. Changes in hip BMD and TL were normally distributed, while heteroscedasticity was not demonstrated in the residual plots of regressions for BMD changes. This allows associations between telomere length and BMD at various sites to be analyzed using multivariate linear models. Covariates of TL and BMD were adjusted in the multivariate regression for both sexes, which included age, BMI, PASE score, daily dietary vitamin D intake, history of smoking, heart diseases, and fracture. In addition, age of menarche, age of menopause, numbers of children, history of breast feeding, and estrogen treatment were also included as covariates for women [36–38]. PASE scores were grouped by deciles in the multivariate analysis. P value less than 0.05 were considered as statistically significant.
Results
At baseline, 963 men and 904 women TL were measured in using real-time quantitative PCR assay. Characteristics of the subjects are shown in Table 1. Women had higher body mass index compared with men. Hip and femoral BMD were higher in men by 16% to 21% compared with women (Table 2). After dividing subjects into three 5-year interval age groups, similar percentage differences between the two sexes were confirmed (Table 3). During follow-up, 75% of men and 81% of women had returned at 4 years. Mean hip BMD loss was 0.007 g/cm2 in men and greater in women (0.016 g/cm2; P < 0.001, Table 2). Similarly, loss in femoral neck BMD was greater in women (P < 0.001). BMD loss was highest in the oldest age group (≥75 year old).
Mean telomere length was 5.40 and 5.95 kb for men and women, respectively. Women had longer telomere than men (P < 0.001; Table 1). There was a general decreasing trend in TL with age in men, and the correlation between TL and age was statistically significant (P = 0.037; Fig. 1). There was no correlation between TL and age in women (P = 0.784).
Table 4 shows the regression coefficients of the association between telomere length, other covariants, and hip and femoral neck BMD at baseline. Age and BMI showed the strongest association in both sexes. In women, age of menopause, age of menarche, estrogen treatment/replacement therapy, and history of previous fracture were also significant covariates. In addition, PASE score and dietary calcium intake were also significantly correlated with BMD at baseline. However, in these multivariate analyses, TL was not associated with BMD in either sex.
To examine if TL could predict bone loss, the analysis was repeated using change in BMD after 4 years (Table 5). Age was the only significant factor associated with change in hip BMD. PASE score was significantly correlated with bone loss at both total hip and femoral neck in men, while history of fracture was also associated with change in hip BMD in women. TL was not associated with change in BMD in either site in men or women.
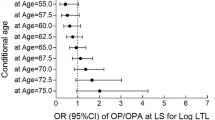
To further explore the association between baseline or loss of BMD and TL, a subgroup analysis was performed. In order to minimize the effect of chronological age, subjects were stratified by sex and age groups of 5 years (65–69, 70–74, and 75 and above). The effect of TL and covariants were analyzed by linear regression in each of these three different age groups. Only a weak association between TL and baseline hip and femoral neck BMD was found in men between 70 and 74 years (Table 6). However, TL only accounts for up to 1.6% of the variance of baseline BMD. The effect of TL on the risk of osteoporosis was explored by logistic regressions with correction for confounding covariates. In both whole sample and age group stratified analyses, TL was not a significant risk factor for osteoporosis of the hip in either sex (Table 7).
Discussion
Two recent clinical studies suggest that leukocyte telomere length could be a marker of biological aging of bone and bone loss [25, 26], and the need for studies with large sample size and prospective design was pointed out. Our report attempted to continue to address this research question using large numbers of men and women examining changes in BMD prospectively. No association between TL and baseline BMD or changes in BMD was observed, although associations between bone loss and other well-known factors such as age, BMI, physical activity, calcium intake, age of menopause, and estrogen use were observed. This conclusion may appear different from two previous studies [25, 26]. However, many factors should be considered in the comparison between this and previous studies. Firstly, the mean age of this cohort was approximately 72 years, compared with the age range of 71–86 years for men in one study [25] and 48 years for women in the other, although one third of women were postmenopausal [26]. In examining association between TL and BMD, large numbers of subjects over a wide age range would be preferable. Our cohort did not include young or middle-aged subjects. This fact may also explain the lack of association between TL and age in our study. Secondly, bone loss may vary among different sites, as a result of factors other than biological aging, such as mechanical factors (body weight, physical activity). The contributory role of TL and these other factors may differ depending on the site. For example, mechanical factors and gravity may exert a greater influence in hip BMD compared with forearm. Only hip BMD was available in this study. In the other studies, the association between TL and BMD was only observed in forearm or spine but not in the hip. The findings of this study confirm the lack of association between TL and hip BMD.
Methodological differences in epidemiological studies of telomeres need to be addressed in comparing findings from different studies. We took advantage of the latest method of qRT-PCR to measure TL with the benefits of high throughput and sensitivity to TL changes comparable to original TRF analysis [33]. This method allows fast and reliable determination of TL length in such a large-scale epidemiological study. However, it has been pointed out that many methodological issues remain unresolved. The accuracy of the qRT-PCR method (used in this study) versus the southern blot analysis (used in the two previous studies [25, 26]) may be inferior as no study comparing the two methods have been carried out. [39]. Ideally in prospective studies, the rate of change in TL should also be measured in addition to the outcome being examined. The duration of follow-up over which TL changes can be detected is uncertain. The ability of current techniques to detect small changes in TL is uncertain.
In spite of the limitations in laboratory methodology, the strength of this study lies in the large numbers of men and women and the longitudinal design allowing changes in BMD to be examined. Furthermore, it represents the first report in a different racial population with a different lifestyle. Although TL may be a biological marker of bone aging, changes in BMD will be differentially affected by many other genetic and environmental factors between populations. Genetic polymorphisms determining the rate of bone loss may differ between different ethnic groups since there are ethnic differences in cross-sectional association between BMD and genetic polymorphisms [40, 41]. Environmental factors may operate differently according to the BMD site being examined. For example, hip BMD may be more affected by lifestyle differences, being a weight-bearing part of the skeleton.
In conclusion, no association was observed between TL and change in hip BMD in Chinese men and women aged 65 years and over. This finding does not necessarily negate the concept of TL as a biological marker of bone aging, since the age range studied was not wide and BMD at the hip site may be more affected by mechanical factors compared with other sites.
Conclusion
Leukocyte telomere length is not associated with baseline hip BMD or rate of bone loss in the elderly population of our study. Age itself appears to be the most important determinant of hip BMD and rate of bone loss in this site rather than telomere length.
References
Vogel T, Bitterling H, Dobler T et al (2006) Contemporary diagnostics and therapy of osteoporosis. Zentralbl Chir 131:401–406
Meunier PJ, Delmas PD, Eastell R et al (1999) Diagnosis and management of osteoporosis in postmenopausal women: clinical guidelines. International Committee for Osteoporosis Clinical Guidelines. Clin Ther 21:1025–1044
Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312:1254–1259
Cauley JA, Hochberg MC, Lui LY et al (2007) Long-term risk of incident vertebral fractures. JAMA 298:2761–2767
Gnudi S, Sitta E, Fiumi N (2007) Bone density and geometry in assessing hip fracture risk in post-menopausal women. Br J Radiol 80:893–897
Cauley JA, Lui LY, Barnes D et al (2008) Successful skeletal aging: a marker of low fracture risk and longevity. The study of osteoporotic fractures (SOF). J Bone Miner Res 24(1):134–143
Leslie WD, Tsang JF, Lix LM (2008) Validation of ten-year fracture risk prediction: a clinical cohort study from the Manitoba Bone Density Program. Bone 43:667–671
Papaioannou A, Kennedy CC, Cranney A et al (2008) Risk factors for low BMD in healthy men age 50 years or older: a systematic review. Osteoporos Int 20:507–518
Aviv A, Levy D, Mangel M (2003) Growth, telomere dynamics and successful and unsuccessful human aging. Mech Ageing Dev 124:829–837
Martens UM, Chavez EA, Poon SSS et al (2000) Accumulation of short telomeres in human fibroblasts prior to replicative senescence. Exp Cell Res 256:291–299
Murnane JP, Sabatier L (2004) Chromosome rearrangements resulting from telomere dysfunction and their role in cancer. Bioessays 26:1164–1174
Pignolo RJ, Suda RK, McMillan EA et al (2008) Defects in telomere maintenance molecules impair osteoblast differentiation and promote osteoporosis. Aging Cell 7:23–31
Goronzy JJ, Fujii H, Weyand CM (2006) Telomeres, immune aging and autoimmunity. Exp Gerontol 41:246–251
van der Harst P, van der Steege G, de Boer RA et al (2007) Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol 49:1459–1464
Magana JJ, Gomez R, Cisneros B et al (2008) Association of interleukin-6 gene polymorphisms with bone mineral density in Mexican women. Arch Med Res 39:618–624
Perez A, Ulla M, Garcia B et al (2008) Genotypes and clinical aspects associated with bone mineral density in Argentine postmenopausal women. J Bone Miner Metab 26:358–365
Styrkarsdottir U, Halldorsson BV, Gretarsdottir S et al (2008) Multiple genetic loci for bone mineral density and fractures. N Engl J Med 358:2355–2365
Tran BN, Nguyen ND, Eisman JA et al (2008) Association between LRP5 polymorphism and bone mineral density: a Bayesian meta-analysis. BMC Med Genet 9:55
Kiel DP, Demissie S, Dupuis J et al (2007) Genome-wide association with bone mass and geometry in the Framingham Heart Study. BMC Med Genet 8(Suppl 1):S14
Richards JB, Rivadeneira F, Inouye M et al (2008) Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 371:1505–1512
Takayanagi H, Sato K, Takaoka A et al (2005) Interplay between interferon and other cytokine systems in bone metabolism. Immunol Rev 208:181–193
Almeida M, Han L, Martin-Millan M et al (2007) Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285–27297
Sanchez-Rodriguez MA, Ruiz-Ramos M, Correa-Munoz E et al (2007) Oxidative stress as a risk factor for osteoporosis in elderly Mexicans as characterized by antioxidant enzymes. BMC Musculoskelet Disord 8:124
Altindag O, Erel O, Soran N et al (2008) Total oxidative/anti-oxidative status and relation to bone mineral density in osteoporosis. Rheumatol Int 28:317–321
Bekaert S, Van Pottelbergh I, De Meyer T et al (2005) Telomere length versus hormonal and bone mineral status in healthy elderly men. Mech Ageing Dev 126:1115–1122
Valdes AM, Richards JB, Gardner JP et al (2007) Telomere length in leukocytes correlates with bone mineral density and is shorter in women with osteoporosis. Osteoporos Int 18:1203–1210
Woo J, Tang NL, Suen E et al (2008) Telomeres and frailty. Mech Ageing Dev 129:642–648
Lau EM, Chan HH, Woo J et al (1996) Normal ranges for vertebral height ratios and prevalence of vertebral fracture in Hong Kong Chinese: a comparison with American Caucasians. J Bone Miner Res 11:1364–1368
Washburn RA, Smith KW, Jette AM et al (1993) The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46:153–162
Cawthon RM (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res 30:e47
Gil ME, Coetzer TL (2004) Real-time quantitative PCR of telomere length. Mol Biotechnol 27:169–172
Norwood D, Dimitrov DS (1998) Sensitive method for measuring telomere lengths by quantifying telomeric DNA content of whole cells. Biotechniques 25:1040–1045
Baird DM (2005) New developments in telomere length analysis. Exp Gerontol 40:363–368
Gardner JP, Kimura M, Chai W et al (2007) Telomere dynamics in macaques and humans. J Gerontol A Biol Sci Med Sci 62:367–374
Strike PW (1991) Statistical methods in laboratory medicine. Chapter 8.9. Measurement and control-precision. Butterworth-Heinemann, Oxford. pp. 273-276.
Ho AY, Kung AW (2005) Determinants of peak bone mineral density and bone area in young women. J Bone Miner Metab 23:470–475
Brouilette SW, Moore JS, McMahon AD et al (2007) Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet 369:107–114
Cherkas LF, Hunkin JL, Kato BS et al (2008) The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med 168:154–158
Aviv A (2008) The epidemiology of human telomeres: faults and promises. J Gerontol Med Sci 63A:979–983
Lau EMC, Choy DT, Li M, Woo J, Chung T, Sham A (2004) The relationship between COLIA 1 polymorphisms (SP I) and COLIA 2 Polymorphisms (ECO R1 and Puv II) with bone mineral density in Chinese men and women. Calcif Tissue Int 75:133–137
Li M, Lau EMC, Woo J (2004) Methylenetetrahydrofolate reductase polymorphism (MTHFR C677T) and bone mineral density in Chinese men and women. Bone 35:1369–1374
Acknowledgments
The study was supported by the Direct Grant from the Chinese University of Hong Kong, the Centre for Nutritional Studies, School of Public Health, and the Hong Kong Jockey Club Charities Foundation.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, N.L.S., Woo, J., Suen, E.W.C. et al. The effect of telomere length, a marker of biological aging, on bone mineral density in elderly population. Osteoporos Int 21, 89–97 (2010). https://doi.org/10.1007/s00198-009-0948-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-009-0948-4