Abstract
Summary
Professional jockeys are routinely exposed to high impact trauma and sustain fractures frequently. We found that jockeys restrict their caloric intake in order to maintain regulation weights, and that bone turnover is high. There are significant health and safety implications for the racing industry.
Introduction
Professional jockeys routinely sustain fractures from high impact falls. Jockeys maintain a low percentage body fat and a low body mass index (BMI) to achieve low weight targets in order to race. We evaluated dietary habits and bone metabolism in jockeys.
Methods
Bone mineral density (BMD) was measured in 27 male jockeys of the 144 jockeys licensed in Ireland. Fourteen (52%) had BMD T score below −1.0, of whom 12 consented to clinical review, nutritional survey, endocrine studies, and bone turnover markers (BTM). BTM were compared to age- and sex-matched controls (n = 16).
Results
BMI was 20.6 ± 1.7 kg/m2; previous fracture frequency was 3.2 ± 2.0 per rider. All had normal endocrine axes. The jockeys' diet as determined by a 7-day dietary recall was deficient in energy, calcium, and vitamin D intake. Compared with the control group, the jockey group had evidence of increased bone turnover.
Conclusions
A substantial proportion of the professional jockeys in Ireland have low–normal BMD, low BMI, and high bone turnover that may result from weight and dietary restrictions. These factors seem to have a deleterious effect on their bone health and predispose the jockeys to a high fracture risk that should be remediated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Professional racing, which is divided into flat racing and jump racing (national hunt racing), is regulated in Ireland by The Turf Club. In Ireland during 2007, 67 flat jockeys, 12 jump jockeys, and 65 apprentice jockeys held full registration as professionals with the Turf Club [1]. Professional jockeys are exposed to unique occupational risks by having to ride racehorses weighing approximately 450 to 550 kg capable of traveling at speeds of over 60 km/h while at a height of over 2 m from the ground [2]. Recent reports from different countries have cataloged the high rate of injuries in jockeys [2–5].
Racehorses are handicapped through weight penalties, with jockeys required to achieve a low percentage body fat and a low body mass index (BMI) to make weights of 52.7 to 64.0 kg for flat racing and 62.0 to 76.0 kg for jump racing in Ireland. This requirement persisted despite the average weight of students entering the Racing Academy Centre of Education increasing from 36.7 kg in 1978 to 45 kg in 2002 [1]. Not unsurprisingly, falls occur regularly with a 5% fall rate per ride and with a 20% injury rate per fall in 2006. Importantly, 25% of all recordable injuries were bone fractures [1]. Though fatalities are relatively rare in professional jockeys, two occurred in Ireland in 2003 that have led efforts by the Turf Club to improve the health and safety of participants in the sport.
A key component of the Turf Club's strategy has been the investigation of physiological function and bone health of professional jockeys. To date, there are no published studies on bone health in jockeys despite the repeated occupational risk of multiple high impact falls with associated fractures [2–5]. Disordered eating is common among jockeys just like other weight-category athletes because the athletes face extreme pressure to maintain unusually low and specific weights, sometimes within a 2.3-kg margin [6]. The main aim of the current study was to investigate dietary habits and bone metabolism in jockeys during the racing season.
Materials and methods
Bone mineral density (BMD) was measured by dual-energy X-ray absorptiometry using Prodigy densitometer (GE Medical system, Lunar, Slough, UK) in a group of 27 jockeys (17 Flat and 10 National Hunt). Jockeys (n = 14) with the lowest BMD—namely, a T-score below −1.0 at either spine or hip—were invited for further study. In total, 12 jockeys (nine Flat and three National Hunt) agreed to partake in the study. All clinical, nutritional, and biochemical investigations were carried out during the racing season.
A physician took a detailed structured medical history with particular emphasis on a history of symptoms of renal disease, liver dysfunction, osteomalacia, hyperthyroidism, pernicious anemia, and fractures. Participants were directly questioned regarding potential use of glucocorticoids, anticonvulsants, diuretics, illegal drugs, and health food/nutritional supplements. A smoking, alcohol, and family history was also taken. A full physical examination, including weight and height measurements, was performed.
Dietary intake was determined by the use of a 7-day food diary. The food intake records were analyzed by WinDiet professional version (WinDiets, the Robert Gordon University, Aberdeen, UK). Results were compared to the recommended dietary allowances for Irish men (Food Safety Authority of Ireland, Dublin, Ireland).
Fasting bloods were drawn for full blood count, urea and electrolytes, calcium, phosphate, magnesium, amylase, creatine kinase, lipids, vitamin B12, folate, and tissue transglutamase antibody. Endocrine axes were investigated by measurement of thyroid-stimulating hormone, prolactin, luteinising hormone, follicular-stimulating hormone (FSH), growth hormone, insulin-like growth factor-1, testosterone, estradiol, and sex hormone binding globulin (SHBG).
Metabolic studies included measurement of serum 25-hydroxyvitamin D (25[OH]D), serum parathyroid hormone (PTH), and BTMs. Jockeys provided samples for BTMs according to our laboratory protocol: Following a fast from midnight, morning blood was collected in serum tubes containing a clot activator, and a second void urine sample was provided. Serum for PTH, 25(OH)D, and bone turnover markers was stored in aliquots at −30°C until analysis. Urine was aliquoted, and samples for bone turnover markers were stored at −30°C.
Serum PTH levels were measured using a commercially available chemiluminescence immunoassay using the Elecsys 1010 platform (Roche, Basel, Switzerland). Intra- and inter-assay coefficients of variation (CV) as reported by the manufacturer were <5.8% and <7.1%, respectively. Serum 25(OH)D levels were measured by a commercially available competitive radioimmunoassay kit (Diasorin, Stillwater, USA). The intra-assay CV at a level of 64.7 nmol/L was <10.8%, and the inter-assay CV was <15%.
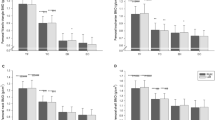
Bone formation markers—bone alkaline phosphatase (bone ALP), procollagen type I N-propeptide (PINP), and intact osteocalcin (OC[1-49])—were measured in serum using commercially available kits. Bone ALP was measured by a radioimmunoassay (Tandem-R Ostase, Beckman Coulter, San Diego, CA, USA). Intra-assay CVs were 6.7%, 4.2%, and 3.7% at concentrations of 13.2, 26.7, and 48.6 μg/L, respectively. The inter-assay CVs were 9.8% and 4.9% at concentrations of 20 and 99 μg/L. PINP was measured by competitive radioimmunoassay (Orion Diagnostica, Espoo, Finland), with intra- and inter-assay CVs of <5% and <7%, respectively. OC[1-49] was measured by sandwich immunoradiometric assay (Cis Bio International, Bagnols/Ceze, France) with intra- and inter-assay CVs of <2% and <6%, respectively.
Bone resorption markers—N-terminal telopeptide of type I collagen (NTX-I) and free deoxypyridinoline crosslinks (fDPD)—were measured in urine on an automated ELISA platform using commercially available kits. NTX-I was measured by competitive inhibition ELISA (Inverness Medical Innovations, Waltham, USA). Intra- and inter-assay CVs were <13% and <7%. fDPD was measured by competitive enzyme immunoassay (Quidel, San Diego, USA). Intra- and inter-assay CVs were <7% and <14%. NTX-I and fDPD were expressed as a ratio with urine creatinine concentration, which was measured by a kinetic Jaffe method.
Results are expressed as mean ± SD. Results of the BTM were compared with results in age-matched young men (n = 16) using an unpaired Mann–Whitney test. Pearson correlation coefficient was employed to test for an association between the between serum 25(OH)D and serum PTH. The level of significance was p < 0.05 for two-tailed tests. Individual BTM results were expressed as Z-scores based on the control group in order to calculate our bone turnover index that is based empirically on Frost's model of bone turnover, namely, the arithmetic mean of the formation rate and the resorption rate [7]. Normality of derived indices in controls was tested by D'Agostino–Pearson normality test. Statistics were performed using GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego, CA, USA).
Results
All jockeys were Irish male Caucasians aged 25.5 ± 5.0 years; controls were aged 24.6 ± 2.2 years. Jockeys' prior fracture frequency was 3.2 ± 2.0 per rider. Fractures were more common in national hunt than flat jockeys. The type of fractures sustained varied in severity from distal radius and clavicle fractures to multiple vertebral and/or pelvic fractures. No jockeys had a fracture at the time of the study. There was no history of use of glucocorticoids, diuretics, or supplements. Four jockeys were smokers, and five consumed moderate amounts of alcohol (less than 21 units of alcohol a week). None had a family history of osteoporosis. On examination, subjects had a BMI of 20.6 ± 1.67 kg/m2. All individuals had a normal physical examination apart from the evidence of previous fractures.
Seven-day food diaries demonstrated certain trends: Compared to recommended ranges listed in brackets, jockeys tended to have lower intakes of energy at 1,760 ± 283 kcal/day (2,000 to 2,500 kcal/day), carbohydrate at 43.9 ± 8.5% (55% to 60%), vitamin D at 1.4 ± 0.8 μg/day (10 μg/day), and calcium at 541 ± 106 mg (800 mg), but higher intakes of protein at 16.2 ± 3.7% (10% to 15%) and fat at 35.3 ± 7.0% (30% to 35%).
All participants had normal hematological and biochemical investigations apart from a one jockey who had a positive transglutaminase antibody result. This subject proceeded to esophagogastroduodenoscopy with duodenal biopsy, which was consistent with a diagnosis of subclinical celiac disease. Jockeys had no evidence of hypogonadism with normal serum levels of LH 1.8 ± 3.5 U/L (N <8 U/L), FSH 5.1 ± 1.95 U/L (N <11 U/L), testosterone 22.6 ± 6.6 nmol/L (N 9–38 nmol/L), and SHBG 41.1 ± 14.6 nmol/L (N 14.9–103 nmol/L).
Serum 25(OH)D levels were very variable; 4 of 12 who had levels below the minimum desirable level of 50 nmol/L [8] were all studied in November; all jockeys studied in the summer had levels in excess of 90 nmol/L. Serum PTH at 30.5 ± 5.5 ng/L was well within the reference range of 12–64 ng/L. There was a trend towards an inverse correlation between serum 25(OH)D and serum PTH that did not achieve significance (r = −0.33; p = ns; Fig. 1). Compared with age-matched controls, both bone resorption markers (NTX-I/creatinine and fDPD/creatinine) and one bone formation marker (PINP) were significantly elevated (Table 1). Derived bone turnover indices for controls all passed the test for normality; in addition, Z-score means were all close to 0 and SDs were close to 1.0, approximating a Gaussian curve. Derived indices were significantly higher in jockeys than controls for all combinations of resorption and formation markers except fDPD with OC(1-49) (Table 1).
Discussion
Increasing attention is being given to the relationship between BMD and risk of high-trauma fractures. A joint report of two prospective US cohort studies in community-dwelling elderly (The Study of Osteoporotic Fractures and The Osteoporotic Fractures in Men Study) reported that high-trauma nonspine fractures are associated with low BMD and increased risk of subsequent fracture in both women and men [9]. It is likely that a similar pattern is seen in jockeys.
In our study, we found that Irish male professional jockeys with low–normal BMD had a high rate of bone fractures and low BMI. Dietary survey demonstrated low energy, low carbohydrate, high fat, low calcium, and low vitamin D intakes. Vitamin D status reflected our experience of seasonal variation in serum 25(OH)D levels in a setting of low dietary intake with excess outdoor lifestyle associated with horse riding [10]. None had secondary hyperparathyroidism, thus excluding marked hypovitaminosis D [11]. However, as a group, jockeys compared to healthy young men had high bone formation markers, high bone resorption markers, and high bone turnover indices. Szulc and Delmas in a recent review gathered the evidence in favor of bone turnover markers as an independent risk factor for fractures [12].
Although there was no evidence of overt secondary hyperparathyroidism, we noted an inverse correlation between vitamin D status and PTH status that did not reach significance probably due to small sample size. However, it is possible that mild hypovitaminosis D, in the wintertime at least, could contribute to the high bone turnover observed given the known relationship between vitamin D status and PTH status in larger studies [13–15]. In Ireland, consumption of fortified milk in the wintertime attenuates but does not prevent the seasonal decline in serum 25(OH)D levels [16]. Recently, Cashman et al. have demonstrated that healthy subjects in wintertime in Ireland need to take a supplemental vitamin D dose of 28 μg/day to achieve a serum 25(OH)D level above 50 mmol/L and a dose of 41 μg/day for a level above 80 mmol/L [17].
In female athletes and ballet dancers, low BMD is well described and has been found to be associated with energy deficiency (expenditure greater than dietary intake), amenorrhea, and low BMD. These three inter-related disorders are referred to as the “female athlete triad” [18]. The jockeys studied differ from this group in so far as they have no evidence of hypogonadism. However, they share similarities in their nutritionally poor diet that allows the maintenance of low body mass despite high energy expenditure while riding.
In men, high impact sports have shown an enduring benefit on BMD [19, 20]. However, low BMD has been described in male professional cyclists. No abnormalities in hormone axes or bone turnover markers were found when cyclists were compared to weight bearing athletes. The low BMD detected in cyclists was attributed to the effect of non-weight bearing exercise on bone metabolism [21]. A similar effect may account for low–normal BMD in professional jockeys. Unlike cyclists, jockeys sustain significant mechanical loading on their bones due to their riding position, termed “riding short” [2], but the effect of this mechanical loading on bone needs further study.
The most likely explanation for high bone turnover in jockeys is the low intake of calcium combined with energy deficiency. Uniquely, jockeys may undergo multiple episodes of rapid weight loss weekly to ensure that they “make the weight”. The commonest form of practice is fasting and dehydration combined with physical activity, other than horse riding, in order to maximize weight loss [22, 23]. This cyclical form of weight wasting may be contributing to the high bone turnover observed in our study [24]. We suggest that the low percent body fat, low BMI, and high bone turnover observed in the jockeys combine to increase fracture risk.
In conclusion, a substantial proportion of professional jockeys in Ireland have high bone turnover that may predispose them to fractures as a result of high impact falls. This abnormality in bone metabolism appears to be a result of self-imposed dietary habits to make the regulation weights and consequently is having a negative impact on the bone health of jockeys. There are major health and safety implications for the multibillion euro racing industry both in Ireland and internationally. On foot of presentation of these findings to The Turf Club, a number of actions have been taken—namely, minimum weight targets pre-racing have been raised, a dietician has been made available to jockeys, and an educational program for apprentice jockeys has been undertaken. The degree of abnormalities in bone turnover raises the question of medical intervention such as calcium and vitamin D supplementation and antiresorptive agents for those with low BMD and high bone turnover. Further studies are required to characterize the extent of bone abnormalities and determine the benefit of medical intervention in this very high risk group.
References
The Turf Club. http://www.turfclub.ie/site/
Turner M, McCrory P, Halley W (2002) Injuries in professional horse racing in Great Britain and the Republic of Ireland during 1992–2000. Br J Sports Med 36:403–409
McCrory P, Turner M, LeMasson B et al (2006) An analysis of injuries resulting from professional horse racing in France during 1991–2001: a comparison with injuries resulting from professional horse racing in Great Britain during 1992–2001. Br J Sports Med 40:614–618
Balendra G, Turner M, McCrory P et al (2007) Injuries in amateur horse racing (point to point racing) in Great Britain and Ireland during 1993–2006. Br J Sports Med 41:162–166
Yim VWT, Yeung JHH, Mak PSK et al (2007) Five year analysis of Jockey Club horse-related injuries presenting to a trauma centre in Hong Kong. Injury 38:98–103
McGoldrick A (2004) Presentation to the safety committee. The Turf Club. http://www.turfclub.ie/site/index.php?option=com_wrapper&Itemid=39
Frost HM (1983) Bone histomorphometry: analysis of trabecular bone dynamics. In: Recker RR (ed) Bone histomorphometry: techniques and interpretation. CRC, Boca Raton, pp 109–131 (2002)
Dawson-Hughes B, Heaney RP, Holick MF et al (2005) Estimates of optimal vitamin D status. Osteoporos Int 16:713–716
Mackey DC, Li-Yung Lui L-Y et al (2007) High-trauma fractures and low bone mineral density in older women and men. JAMA 298:2381–2388
McKenna MJ (1992) Differences of vitamin D status between various countries in young adults and the elderly. Amer J Med 93:69–77
McKenna MJ, Freaney R (1998) Secondary hyperparathyroidism in the elderly: means to defining hypovitaminosis D. Osteoporos Int 8(Suppl 2):S3–S6
Szulc P, Delmas PD (2008) Biochemical markers of bone turnover: potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos Int 19:1683–1704
Chapuy MC, Schott AM, Garnero P, Hans D, Delmas PD, Meunier PJ (1996) Healthy elderly French women living at home have secondary hyperparathyroidism and high bone turnover in winter. EPIDOS Study Group. J Clin Endocrinol Metab 81:1129–1133
Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE (2005) Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 90:3215–3224
Malabanan A, Veronikis IE, Holick MF (1998) Redefining vitamin D insufficiency. Lancet 351:805–806
McKenna MJ, Freaney R, Byrne P, McBrinn Y, Murray B, Kelly M, Donne B, O'Brien M (1995) Safety and efficacy of increasing wintertime vitamin D and calcium intake by milk fortification. Q J Med 8:895–898
Cashman KD, Hill TR, Lucey AJ, Taylor N, Seamans KM, Muldowney S, Fitzgerald AP, Flynn A, Barnes MS, Horigan G, Bonham MP, Duffy EM, Strain JJ, Wallace JM, Kiely M (2008) Estimation of the dietary requirement for vitamin D in healthy adults. Am J Clin Nutr 88:1535–1542
Warren MP, Brooks-Gunn J, Fox RP et al (2002) Osteopenia in exercise-associated amenorrhea using ballet dancers as a model: a longitudinal study. J Clin Endocrinol Metab 87:3162–3168
Andreoli A, Monteleone M, Van Loan M et al (2001) Effects of different sports on bone density and muscle mass in highly trained athletes. Med Sci Sports Exerc 33:507–511
Nordstorm A, Olsson T, Nordstorm P (2006) Sustained benefits from previous physical activity on bone mineral density in males. J Clin Endocrinol Metab 91:2600–2604
Rector RS, Rogers R, Ruebel M et al (2008) Participation in road cycling vs running is associated with lower bone mineral density in men. Metabolism 57:226–232
Moore JM, Timperio AF, Crawford DA et al (2002) Weight management and weight loss strategies of professional jockeys. Int J Sport Nut Exercise Metab 12:1–13
Leyon MA, Wall C (2002) New Zealand jockey's dietary habits and their potential impact on health. Int J Sport Nut Exercise Metab 12:220–237
Villareal DT, Fontana L, Weiss EP et al (2006) Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss. Arch Intern Med 166:2502–2510
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Waldron-Lynch, F., Murray, B.F., Brady, J.J. et al. High bone turnover in Irish professional jockeys. Osteoporos Int 21, 521–525 (2010). https://doi.org/10.1007/s00198-009-0887-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-009-0887-0