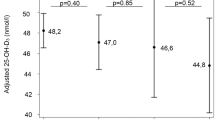Abstract
Summary
Serum 25(OH)D levels decline without sunlight exposure. We studied 120 expeditioners to Antarctica to determine the skeletal and hormonal responses to sunlight deprivation. With emerging vitamin D insufficiency, serum calcium decreased, PTH increased, and bone loss at the proximal femur was observed. Baseline serum 25(OH)D levels >100 nmol/L prevented vitamin D insufficiency.
Introduction
Vitamin D stores deplete without adequate sunlight exposure unless supplementation is provided. We studied 120 healthy adults who spent a year in Antarctica as a model for sunlight deprivation to define the timing and magnitude of the skeletal and hormonal responses to emerging vitamin D insufficiency.
Methods
Fasting blood samples were assessed at baseline, 6 and 12 months for serum 25-hydroxyvitamin D (25(OH)D), osteocalcin (OC), bone formation (P1NP) and resorption (CTx), PTH and calcium. Lumbar spine and proximal femur BMD was measured using DXA. Differences over time were determined using repeated measures ANOVA. Percent changes were expressed as (Δ value/(value A + value B)/2) × 100. Relationships between outcome measures were determined using Spearman’s correlations.
Results
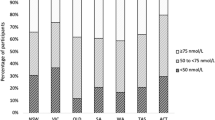
Vitamin D insufficiency (<50 nmol/L) was observed in 85% of expeditioners by 6 months when serum calcium decreased and PTH increased (p < 0.01). By 12 months, OC increased by 7.4 ± 3.0% (p < 0.05), and BMD decreased by 1.0 ± 2.0% at the total proximal femur (p < 0.05). For those with vitamin D sufficiency at baseline (>50 nmol/L), sunlight deprivation produced vitamin D insufficiency within 4 months unless baseline values were >100 nmol/L.
Conclusion
Supplementation may be necessary for expeditioners with limited access to UV light.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D stores become depleted without adequate sunlight exposure unless supplementation is provided. For this reason, vitamin D insufficiency (serum 25(OH)D < 50 nmol/L) is common during winter in many countries, even in those where sunlight exposure is plentiful in summer [1–4]. Little is known about the rate of reduction in serum 25(OH)D during sunlight deprivation and its effects on serum calcium, the emergence of secondary hyperparathyroidism, increased bone remodeling, and subsequent bone loss.
Although bone loss may be partly reversed following re-exposure to sunlight because the remodeling space deficits produced by accelerated remodeling as deficiency appears is reversed as remodeling slows with repletion, there may be an irreversible component in adults, particularly older adults because of the negative bone balance produced by the reduced volume of bone formed by each basic multicellular unit (BMU) than is resorbed by each BMU [5, 6]. Repeated periods of prolonged sunlight deprivation during adulthood may thus result in permanent bone loss [7].
Expeditioners to Antarctica provide a model for the skeletal and bone metabolic responses to sunlight deprivation. UV exposure from March to August is negligible, and marginal in the shouldering months, which corresponds to autumn (March–May), winter (June–August), and the commencement of spring (September–November) in the southern hemisphere [8]. Extreme conditions also limit skin exposure. Reductions in 25(OH)D have been reported in Antarctic expeditioners; however, skeletal changes are inconclusive [9–12]. We studied Australian Antarctic expeditioners to determine the temporal pattern and magnitude of emerging vitamin D insufficiency, changes in circulating measures of bone remodeling, and rates of bone loss during long-term sunlight deprivation.
Materials and methods
This prospective study involved 120 healthy adults (19 females, 101 males) free of disease known to affect bone who spent a year at Australian Antarctic or sub-Antarctic coastal stations for the 2004–2005 (n = 71) or 2005–2006 (n = 49) seasons. Departures to Antarctica were scheduled between October and March, depending on station destination. Baseline data was collected before departure at the Australian Antarctic Division (Tasmania, Australia) or during the ship voyage to Antarctica. At baseline, each participant completed a standard medical questionnaire. Height (±0.1 cm) was measured using a stadiometer (Medizintechnic, Germany) and weight (±0.1 kg) measured on a beam scale (Wedderburn, UK). Fasting blood samples were taken at baseline, then as near as possible to quarterly (2004–2005 season) or biannually (2005–2006 season). All samples were stored at –80°C to be assayed in single batches. Dietary intake of macronutrients and calcium was assessed at 6 months (n = 107) using a 3-day weighed food diary, and analyzed using FoodWorks (version 2.10, Xyris Software). Dietary vitamin D was negligible, as foods were not fortified and deep-sea fish and other vitamin D rich sources not staples. Bone mineral density (BMD) at the total proximal femur (neck, trochanter, and proximal shaft) and lumbar spine (L1–4) was assessed at the Menzies Research Institute (Tasmania, Australia) before and after the 2005–2006 expedition using DXA (Hologic Delphi W Version 11.1, CV = 1%) Hologic, Waltham, MA, USA.
Serum 25(OH)D was assessed using chemiluminescent immunoassay (CLIA; Liason, DiaSorin, Stillwater, USA). Serum 25(OH)D levels of <50 nmol/L are insufficient based on Australian recommendations [13]. Serum N-Mid Osteocalcin (OC), total procollegen type 1 amino-terminal propeptide (PINP), and c-terminal telopeptide (CTx) were measured using the electrochemiluminescence immunoassay (Elecsys 1010 Analytics, Roche Diagnostics, Germany) [14] and intact PTH assessed using CLIA (DPC Immulite 2000, Los Angeles, USA). Serum calcium was assessed by indirect potentiometry (SYNCHRON LX, Beckman Coulter USA). The intra- and interassay CVs for serum measures were 7–13% [15]. Due to late arrivals to, or early departures from Antarctica (n = 22), dropouts (n = 3), and field trips (n = 2), full serum data sets were available from 93 of 120 participants. A total of 38 baseline and final BMD assessments were performed during the 2005–2006 expedition (84% males). One female participant consumed a calcium supplement, four were postmenopausal, two were taking the OCP, and none were on HRT. Mean age for women was 36.7 ± 9.6 years. Approval for the study was obtained from the Australian Antarctic Division and Austin Hospital Human Research Ethics Committees.
Comparisons between time points (0, 6, 12 months) were determined using repeated measures analysis of variance (n = 93). Δ time = age at time B − age at time A. Percent changes were calculated as (Δ value/(value A + value B)/2) × 100, accounting for regression to the mean and producing a conservative estimate of changes [16]. Correlations between outcome measures were determined using Spearman’s correlations to account for skewness of some variables. A curve linear regression was used for changes in serum 25(OH)D [3]. A sample of 35 was needed to detect seasonal differences in 25(OH)D and 32 to detect changes in BMD with 80% power using a two-tailed test at p < 0.05 [17]. Data were analyzed using StatView (Version 5.0, SAS Institute, Berkeley, USA). Mean ± SD are presented unless otherwise stated.
Results
Vitamin D insufficiency (<50 nmol/L) was observed at baseline in 44% of expeditioners and 85% by mid-expedition (6 months), when serum calcium decreased, and PTH increased (p < 0.01), with 10% of participants having PTH levels >7.2 pmol/L (Tables 1 and 2). By 12 months, serum OC increased (p < 0.05), BMD decreased by 1.0 ± 2.0% at the total proximal femur (p < 0.05), and 25(OH)D remained 37% below baseline (Table 2 and Fig. 1). There was a trend toward increasing PTH levels with time since baseline measure (r = 0.12, p < 0.07), which was significant for those with vitamin D insufficiency at baseline (r = 0.18, p < 0.05). Those with vitamin D insufficiency at baseline were younger and had higher baseline serum calcium levels (p < 0.05) (Table 1). Vitamin D insufficiency at baseline was not related to usual residency, occupation, number of prior expeditions, weight, or BMI (data not shown). Those who experienced bone loss at the total proximal femur had higher baseline OC levels (23.2 ± 1.3 vs 19.0 ± 1.3 ng/ml, p < 0.05) and lower calcium intakes (678 ± 49 vs 1,189 ± 345 mg, p < 0.07) compared to those who did not, but they did not differ by age (41.5 ± 9.9 vs 44.1 ± 9.2 years) or baseline 25(OH)D levels (63.4 ± 23.3 vs 53.9 ± 22.7 nmol/L). Those with high rates of bone remodeling tended to lose more bone at the total proximal femur (Table 3). For those who were vitamin D replete at baseline (serum 25(OH)D > 50 nmol/L), sunlight deprivation produced vitamin D insufficiency within 4 months unless starting values were greater than 100 nmol/L (Fig. 2).
Discussion
The conditions experienced by Antarctic expeditioners: limited UV light, protective clothing, unfortified food, and lack of supplementation, provides a model for the study of skeletal and metabolic effects of sunlight deprivation. For all expeditioners, serum 25(OH)D and serum calcium decreased and PTH increased within 6 months of limited UV exposure, but the increase in bone turnover was observed about 6 months later. A reduction in total proximal femur, but not lumbar spine BMD, was observed following the expedition. Those who experienced bone loss had higher initial bone turnover rates and lower dietary calcium intakes. Bone loss was associated with higher bone remodeling. In vitamin D replete adults, sunlight deprivation resulted in insufficiency within 4 months, unless baseline values were greater than 100 nmol/L.
In countries without routine food fortification, periods of immobilization, hospitalization, or institutionalization, especially in the elderly may cause a similar skeletal response to that observed in expeditioners [18]. The effectiveness of sunlight exposure or vitamin D supplementation to correct vitamin D deficiency and reduce fracture risk in the elderly living in aged care is documented, but less is known about the efficacy of supplementation and food fortification in the general population [19, 20]. Controlled sunlight exposure at appropriate times is an effective means of maintaining adequate serum 25(OH)D levels, but is difficult to monitor to prevent overexposure which may, in turn, increase skin cancer risk [21].
Despite the rapid reduction in serum 25(OH)D in the first 6 months and 62% of expeditioners reporting increases in PTH levels, only 10% of expeditioners demonstrated PTH levels above the reference range (>7.2 pmol/L), and serum calcium remained within normal limits with no detectable change to markers of bone remodeling. An increase in OC was observed at 12 months in this and previous Antarctic expeditioners perhaps indicating a new steady state at a higher remodeling rate at this time [11]. Vitamin D supplementation is not routine; therefore, the stabilization of serum 25(OH)D at 12 months may have resulted from UV exposure during the warmer months (November–January) [22].
Total proximal femur BMD, but not lumbar spine bone loss, was observed suggesting that bone loss may proceed by cortical rather than trabecular bone loss due to secondary hyperparathyroidism, as previous studies have reported that cortical bone loss was not always reversed after correction of hyperparathyroidism [5]. Those with bone loss had higher bone turnover at baseline. Baseline OC was associated with higher rates of bone loss over 5 years in older women [23]. Moreover, those who demonstrated bone loss had lower dietary calcium intakes; therefore, supplementation of these individuals may be beneficial in suppressing bone turnover [24]. Despite the high prevalence of vitamin D insufficiency (<50 nmol/L), an association between baseline or change in vitamin D levels and bone loss was not observed, which may be due to the low incidence (<1%) of severe deficiency (<12.5 nmol/L) in this group, or the error associated with serum 25(OH)D measurements [25].
The bone loss observed at the total proximal femur is similar to that reported by others during winter [17]. Wintertime bone loss may be higher in groups living at moderate to high latitudes and in persons with limited sunlight opportunities such as veiled immigrant women, persons with very dark skin, or institutionalized elderly unless they are supplemented. For example in Hobart, Australia (latitude 39°), it is estimated that in winter, to produce sufficient serum 25(OH)D, more than 55 min of UV exposure is needed to produce one sixth–one third MED for people with white skin (Fitzpatrick classification II) with 15% of the body exposed [26]. This is unlikely to be achieved in high-risk groups, and supplementation is an option in such cases [27].
A serum 25(OH)D level >100 nmol/L has been suggested as optimal for numerous health outcomes and was estimated to prevent insufficiency after 4 months of limited UV exposure. However, this level is not often achieved, even during summer [3, 28]. Adequate sunlight exposure during winter is unlikely in regions at latitudes greater than 37°, but wintertime vitamin D insufficiency is also observed in countries with unlimited sunshine and routine food fortification, supporting a global trend toward vitamin D insufficiency [3, 4, 29]. Factors that may contribute to vitamin D insufficiency include the aging of the population, sun protection, and religious practices, migration of people from equatorial regions to more extreme latitudes, and a reduction in sunlight exposure due to pollution [13].
This study is limited by the small sample of women and the wide age range preventing sex comparisons or investigation of the influence of menopausal status on the observed responses, or if lumbar spine bone loss occurred in women. The bone loss at the total proximal femur was small and of uncertain biological significance. Moreover, follow-up bone density data was unavailable; therefore, the permanency of the bone loss cannot be confirmed. Following up expeditioners in the future may elucidate this.
Vitamin D insufficiency is likely after 4 months of limited sunlight exposure, especially for those with initial serum 25(OH)D levels less than 100 nmol/L. If bone loss is only partly reversed after prolonged sunlight deprivation, then BMD may remain reduced and results in an increased fracture risk. Baseline assessments prior to prolonged sunlight deprivation are advisable, and supplementation may be necessary for those with serum 25(OH)D levels < 100 nmol/L and limited access to UV light.
References
Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T (2001) A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab 86(3):1212–1221
Rapuri PB, Kinyamu HK, Gallagher JC, Haynatzka V (2002) Seasonal changes in calciotropic hormones, bone markers, and bone mineral density in elderly women. J Clin Endocrinol Metab 87(5):2024–2032
Pasco JA, Henry MJ, Kotowicz MA, Sanders KM, Seeman E, Pasco JR, Schneider HG, Nicholson GC (2004) Seasonal periodicity of serum vitamin D and parathyroid hormone, bone resorption, and fractures: the Geelong Osteoporosis Study. J Bone Miner Res 19(5):752–758
Levis S, Gomez A, Jimenez C, Veras L, Ma F, Lai S, Hollis B, Roos BA (2005) Vitamin d deficiency and seasonal variation in an adult South Florida population. J Clin Endocrinol Metab 90(3):1557–1562
Parfitt AM, Rao DS, Stanciu J, Villanueva AR, Kleerekoper M, Frame B (1985) Irreversible bone loss in osteomalacia. Comparison of radial photon absorptiometry with iliac bone histomorphometry during treatment. J Clin Invest 76(6):2403–2412
Holick MF (1994) McCollum Award Lecture, 1994: vitamin D—new horizons for the 21st century. Am J Clin Nutr 60(4):619–6130
Seeman E (2003) Reduced bone formation and increased bone resorption: rational targets for the treatment of osteoporosis. Osteoporos Int 14(Suppl 3):S2–S8
Pitson GA, Lugg DJ, Roy CR (1996) Effect of seasonal ultraviolet radiation fluctuations on vitamin D homeostasis during an Antarctic expedition. Eur J Appl Physiol Occup Physiol 72(3):231–234
Oliveri B, Zeni S, Lorenzetti MP, Aguilar G, Mautalen C (1999) Effect of one year residence in Antarctica on bone mineral metabolism and body composition. Eur J Clin Nutr 53(2):88–91
Oliveri MB, Mautalen C, Bustamante L, Gomez Garcia V (1994) Serum levels of 25-hydroxyvitamin D in a year of residence on the Antarctic continent. Eur J Clin Nutr 48(6):397–401
Yonei T, Hagino H, Katagiri H, Kishimoto H (1999) Bone metabolic changes in Antarctic wintering team members. Bone 24(2):145–150
Zerath E, Holy X, Gaud R, Schmitt D (1999) Decreased serum levels of 1,25-(OH)2 vitamin D during 1 year of sunlight deprivation in the Antarctic. Eur J Appl Physiol Occup Physiol 79(2):141–147
Working group of the Australian and New Zealand Bone and Mineral Society, Endocrine Society of Australia and Osteoporosis Australia (2005) Vitamin D and adult bone health in Australia and New Zealand: a position statement. Med J Aust 182(6):281–285
Garnero P, Borel O, Delmas PD (2001) Evaluation of a fully automated serum assay for C-terminal cross-linking telopeptide of type I collagen in osteoporosis. Clin Chem 47(4):694–702
Bass S, Delmas PD, Pearce G, Hendrich E, Tabensky A, Seeman E (1999) The differing tempo of growth in bone size, mass, and density in girls is region-specific. [see comments.]. J Clin Invest 104(6):795–804
Hopper JL, Seeman E (1994) The bone density of female twins discordant for tobacco use. N Engl J Med 330(6):387–392
Storm D, Eslin R, Porter ES, Musgrave K, Vereault D, Patton C, Kessenich C, Mohan S, Chen T, Holick MF, Rosen CJ (1998) Calcium supplementation prevents seasonal bone loss and changes in biochemical markers of bone turnover in elderly New England women: a randomized placebo-controlled trial. J Clin Endocrinol Metab 83(11):3817–3825
Lips P (2001) Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 22(4):477–501
Sato Y, Iwamoto J, Kanoko T, Satoh K (2005) Amelioration of osteoporosis and hypovitaminosis D by sunlight exposure in hospitalized, elderly women with Alzheimer’s disease: a randomized controlled trial. J Bone Miner Res 20(8):1327–1333
Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ (1992) Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 327(23):1637–1642
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357(3):266–281
Gies P, Javorniczky J, Roy C, Ayton J, Watzl R, Cooley H, Kingston M (2006) Measurement of the UVR Exposures of Expeditioners on Antarctic Resupply Voyages National Institute of Water and Atmospheric Research UV Workshop-UV Radiation and its Effects:an Update, Dunedin, New Zealand
Lenora J, Ivaska KK, Obrant KJ, Gerdhem P (2007) Prediction of bone loss using biochemical markers of bone turnover. Osteoporos Int 18(9):1297–1305
Zhu K, Devine A, Dick IM, Wilson SG, Prince RL (2007) Effects of calcium and vitamin D supplementation on hip bone mineral density and calcium-related analytes in elderly ambulatory Australian women: a 5-year randomized controlled trial. J Clin Endocrinol Metab 93:852–860
Garnero P, Munoz F, Sornay-Rendu E, Delmas PD (2007) Associations of vitamin D status with bone mineral density, bone turnover, bone loss and fracture risk in healthy postmenopausal women. The OFELY study. Bone 40(3):716–722
Samanek AJ, Croager EJ, Giesfor Skin Cancer Prevention P, Milne E, Prince R, McMichael AJ, Lucas RM, Slevin T (2006) Estimates of beneficial and harmful sun exposure times during the year for major Australian population centres. Med J Aust 184(7):338–341
Grover SR, Morley R (2001) Vitamin D deficiency in veiled or dark-skinned pregnant women. Med J Aust 175(5):251–252
Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B (2006) Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84(1):18–28
Vecino-Vecino C, Gratton M, Kremer R, Rodriguez-Manas L, Duque G (2006) Seasonal variance in serum levels of vitamin d determines a compensatory response by parathyroid hormone: study in an ambulatory elderly population in Quebec. Gerontology 52(1):33–39
Acknowledgements
The authors thank Robin Taylor, Dr. Roland Watzl and Melissa Kingston (AAD), research nurses Kylie King and Judy Tan (Austin Health), Antarctic medical practitioners Drs. Tanya Kelly, Andy Williams, Malcolm Arnold, John Birss, Graham Denyer, James Double, Jim Bumak, and Lloyd Fletcher, Drs. Colin Roy, Stuart Henderson, and Peter Gies (ARPANSA) for use of UV data, Suzanne Sparshott, Rose Ford, and Furley Johston (Menzies) and Jane Karpavicius, Tanya Mewbury, and Mary-Kate Inkster (Monash University).
Funding
This project was support by Australian Antarctic Science grants.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iuliano-Burns, S., Wang, X.F., Ayton, J. et al. Skeletal and hormonal responses to sunlight deprivation in Antarctic expeditioners. Osteoporos Int 20, 1523–1528 (2009). https://doi.org/10.1007/s00198-008-0830-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-008-0830-9