Abstract
Summary
Magnetic resonance imaging was used to show that children with quadriplegic cerebral palsy and unable to ambulate independently compared to typically developing children have a remarkably underdeveloped femoral midshaft as indicated by a very thin diameter, a very thin cortical wall, and very low strength estimates.
Introduction
The femoral shaft is very susceptible to fracture in children with quadriplegic cerebral palsy (QCP); however, its structure and strength have not been evaluated.
Methods
The volume and width of the middle third of the femur (midfemur) and its cortical wall and medullary cavity were assessed in children with QCP and unable to ambulate independently and typically developing children (n = 10/group) using magnetic resonance imaging (MRI). Estimates of cross-sectional moment of inertia (CSMI), section modulus (Z), and polar moment of inertia (J) were also determined.
Results
Total volume of the midfemur and volume of its cortical wall and medullary cavity were substantially lower in children with QCP than controls (51–55%; p < 0.001). In addition, the total midfemur, its medullary cavity and the anterior, posterior, and lateral sections of its cortical wall were thinner (27–43%) in children with QCP (p < 0.001). The midfemur in children with QCP also had remarkably lower CSMI, Z, and J (60–71%; p < 0.001).
Conclusions
Children with QCP who lack the ability to ambulate independently have midfemurs that are very thin with very thin cortical walls and very low estimated strength. The disparity can be detected using MRI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral palsy is a disorder that impairs coordination and limits mobility. A major secondary complication of cerebral palsy, especially in individuals with quadriplegic cerebral palsy (QCP) who are unable to ambulate independently, is a high incidence of fracture in the lower limbs [1, 2]. There is evidence that 28% of children with QCP who are non-ambulatory will experience a skeletal fracture before reaching adulthood [3], with more than 80% of the fractures occurring in the lower extremities [2]. The high fracture rate is associated with an extremely low areal bone mineral density (aBMD) [3, 4].
Although aBMD is the single best in vivo indicator of bone strength widely available, other features of the skeleton should be considered when evaluating fracture risk. There is a substantial overlap in aBMD in those who do and do not experience a skeletal fracture [5–8]. It has been suggested that up to 50% of the variance in bone strength is explained by other features of the skeleton, such as its cortical structure in the shafts of long bones [9] and trabecular bone microarchitecture at sites with a high composition of trabecular bone [5–7, 10–12]. This notion is supported by studies demonstrating an improvement in the prediction of strength and fracture when bone mineral measures are combined with structural measures [7, 9, 13].
Magnetic resonance imaging (MRI) and computed tomography (CT) allow for accurate evaluation of the three-dimensional structure of bone in vivo [14, 15]. Both assessment techniques have advantages when studying bone in humans. Computed tomography is attractive because image acquisition is rapid. In addition, smaller, more affordable units can be housed in a laboratory or transported between testing sites. However, because CT exposes individuals to ionizing radiation, most investigators limit their studies to a single slice per bone site. Recent evidence suggests that this approach may be problematic in children with cerebral palsy because of substantial variability with a minor offset in slice positioning [16]. Furthermore, most peripheral CT units have a small bore that limits the evaluation of bone structure to the forearm and the tibia. Most fractures in children with QCP occur in the femur [2]. To date, no studies have evaluated bone structure and strength in the midshaft of a long bone in children with QCP.
The primary purpose of the present study was to compare the bone structure and strength of the midfemur in a group of children with QCP who are unable to ambulate independently and a group of typically developing children using MRI. It was hypothesized that the total midfemur and its cortical and medullary compartments would be smaller and thinner, and the estimated strength would be much lower in the children with QCP than the typically developing controls.
Materials and methods
Subjects
Twelve children with QCP (n = 8 girls and 4 boys) who could not ambulate independently and between 5 and 12 years of age were recruited from the AI duPont Hospital for Children in Wilmington, DE, USA. Ten typically developing children matched to children with QCP for age, pubertal development, gender, and race, between the 10th and 90th percentiles for height, body mass, and body mass index (BMI) [17], with no history of chronic medication use and with no previous fracture in at least one lower extremity were recruited to serve as controls. Testing procedures were in accordance with the ethical standards of the institutions on human experimentation. The study was approved by the Institutional Review Boards at the University of Delaware and the AI duPont Hospital for Children. Parents gave written consent and children, if able, gave written assent before any testing was conducted.
Anthropometrics
Height and body mass were assessed while the children were wearing minimal clothing and were without shoes or braces. In children with QCP, height was estimated from forearm length [18]. In typically developing children, height was measured to the nearest 0.1 cm using a stadiometer mounted against a wall. Body mass was assessed in both groups using a digital wheelchair scale to the nearest 0.2 kg (Detecto 6550, Cardinal Scale, Webb City, MO, USA). Height, body mass, and BMI percentiles were determined using normative graphs published by the Center for Disease Control [19].
Tanner staging
Pubertal maturity was assessed by a physician assistant using the Tanner staging technique [20]. In boys, signs of pubic hair and testicular/penis development were assessed. In girls, signs of pubic hair and breast development were assessed. The Tanner stage rating system ranges from 1 to 5, with 1 indicating no signs of pubertal development and 5 indicating full development.
Gross motor function classification
A physician assessed gross motor function of children with QCP using the gross motor functional classification (GMFC) system [21]. The GMFC scale ranges from 1 to 5 and provides a way to standardize the range of mobility in individuals with cerebral palsy. Children with moderate to severe forms of cerebral palsy were recruited to participate in this study. Children who achieved independent sitting by 4 years of age and ambulated with assistive devices and/or the aid of an adult were classified as GMFC 3. Children who were ambulating minimally, even with assistance, and had poor sitting trunk control were classified as GMFC 4. Children who lacked independent motor function, even for basic antigravity control, were classified as GMFC 5.
Magnetic resonance imaging
The geometric structure and strength of the middle third of the femur in the more affected side of children with QCP and the nondominant side in typically developing children were estimated using MRI (GE 1.5 T, Milwaukee, WI, USA). Prior to imaging, children were immobilized from the waist down using the BodyFIX (Medical Intelligence, Schwabműnchen, GER), as previously described [4]. The femur was then identified using a three-plane localizer. The region of interest box was carefully positioned using the distal end of the femoral head and the proximal end of the femoral condyles to indicate the top and bottom, respectively, of the region of interest box. Using the localizer in the coronal plane, the region of interest box was rotated so its midline was placed alongside the lateral portion of the femoral head and ran through the center of the interchondalar notch between the femoral condyles. Using the localizer in the sagittal plane, the region of interest box was positioned so its midline went through the center of the femoral head and the center of the medial condyle. Axial images (1-cm thick separated by 0.5-cm spacing) of the entire nondominant femur were collected using a torso PA coil and the following protocol: TR = 750, TE = 14, FOV = 16, 1 NEX, Phase = 512; frequency = 512. In some children, motion was detected in the initial scan, and a second scan was needed to obtain images of good quality.
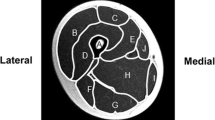
Images at the level of the middle third of the femur (seven to 11 images) were analyzed for measures of geometric structure and strength using custom software designed in-house with Interactive Data Language (IDL; Research Systems, Boulder, CO, USA). Images were first filtered with a median filter to reduce pixel noise. The filtered images were then subjected to automatic segmentation with a fuzzy C-means clustering algorithm. Using the software, cortical bone and medullary cavity were identified and summed to determine their cross-sectional areas. Their volumes were quantified by accounting for image thickness and spacing between images. Middle images were multiplied by 1.5 to account for the 1.0-cm-slice thickness of each image and the 0.5-cm gap between images. Images that included the proximal and distal ends of the region of interest were multiplied by an appropriate correction factor (<1.5), so the entire image set represented the middle third of the femur. Volume of the total mid-femur was determined by summing cortical volume and medullary volume.
Width of the total bone and the medullary cavity in the anterior–posterior and medial–lateral directions and width of the cortical wall’s anterior, posterior, medial and lateral aspects were determined using the segmented images and an automated procedure. Cross-sectional moment of inertia of the mid-femur in the anterior–posterior (CSMIap) and medial–lateral (CSMIml) directions were determined using the parallel-axis theorem [22]. Polar moment of inertia (J) was calculated by summing CSMIap and CSMIml. Section modulus in the anterior–posterior and medial–lateral directions (Z ap and Z ml, respectively) was calculated by dividing CSMIap and CSMIml by the furthest distance from the neutral axis in the anterior–posterior and medial–lateral directions, respectively.
The repeatability of measures of geometric structure and strength was evaluated in three children with QCP and six typically developing children (5 to 12 years) tested twice on two different days or on the same day after repositioning. Coefficients of variation were 1.4% for cortical volume, 2.6% for CSMIap, 1.5% for CSMIml, 1.7% for J, 1.4% for Z ap, and 1.4% for Z ml.
Physical activity
To provide an objective measure of the children’s level of physical activity, an activity monitor (Actical; MiniMitter, Sunriver, OR, USA) was worn around the waist on the more affected side in children with QCP and on the nondominant side in typically developing children. The monitor was positioned so it was most sensitive to vertical movements in the torso. The monitor was worn continuously on three week days and one weekend day. The total number of activity counts were recorded every 15 s [23]. Four days of data from an activity monitor has good reliability and validity (37).
Dietary intake
During 3 days in which the activity monitor was worn (i.e., 2 days during the week and 1 day on the weekend), dietary and supplement intake were recorded by subject parents, with assistance from the subject (if able). To facilitate accurate food estimates, each subject and their parent received a list of serving size estimates based on comparisons to everyday objects (e.g., a serving of meat is approximately the size of a deck of cards) [24]. Total energy intake and calcium intake were estimated from the diet records using the USDA Food and Nutrient Database for Dietary Studies, 1.0 [25].
Statistics
Data were analyzed using SPSS, version 15.0 (Chicago, IL, USA). Group differences in physical characteristics and measures of bone structure and strength were determined using independent t tests if data were normally distributed (skewness and kurtosis <2.00) and Mann Whitney U tests if the data were not normally distributed (skewness and kurtosis >2.00). The magnitude of effects was assessed using Cohen’s d (d), with values of 0.2, 0.5, and 0.8 indicating small, medium, and large effects [26]. An alpha level of 0.05 was used for all significance tests. All data are reported as means ± SD. If data were not normally distributed, medians and ranges are also reported in the text.
Results
All data were considered normally distributed (skewness and kurtosis < 2.00) except calcium intake and total physical activity counts. One girl and one boy with QCP could not complete testing because of fear of the MRI scanner. Characteristics of subjects who completed the study are reported in Table 1. Two children with QCP had a GMFC equal to 3, and the remainder had a GMFC equal to 5. There were no group differences in age, Tanner stage, or BMI. Children with QCP had significantly lower height (13%, d = 1.75, p = 0.001), femur length (16%, d = 1.32, p = 0.028), and body mass (25%, d = 1.06, p = 0.038). Seven children in each group were prepubertal, and three children in each group showed signs of pubertal development. Total physical activity counts were 5.6-fold higher in typically developing children than children with QCP (d = 2.03, p < 0.001; QCP median = 68,351 counts/day, range = 6531 to 527,759 counts/day; control median = 568,350 counts/d, range = 243,588 to 1,612,162 counts/day); however, there were no group differences in calcium intake (d = 0.296, p = 0.870; QCP median = 716 g/day, range = 496 to 2,416 g/day; control median = 797 g/day, range = 501 to 1,134 g/day), or energy intake (d = 0.323, p = 0.515).
Measures of bone volume are reported in Fig. 1. Children with QCP vs. typically developing children had 54% lower total bone volume (d = 2.92, p < 0.001), 55% lower cortical volume (d = 3.09, p < 0.001) and 51% lower medullary volume (d = 2.28, p < 0.001) in the midfemur. Measures of bone width are reported in Fig. 2. The total midfemur was 29% thinner in the anterior–posterior direction (d = 2.97, p < 0.001) and 28% thinner in the medial–lateral direction (d = 3.04, p < 0.001) in the children with QCP. The children with QCP vs. controls also had a cortical wall that was 28, 32, and 43% thinner in the anterior (d = 1.84, p = 0.001), posterior (d = 2.17, p < 0.001), and lateral (d = 1.94, p < 0.001) aspects of the midfemur, respectively, but no difference was detected in the medial aspect (d = 0.31, p = 0.499). Furthermore, the medullary cavity was 27% thinner in the anterior–posterior direction (d = 2.25, p < 0.001) and 28% thinner in the medial–lateral direction (d = 2.59, p < 0.001) in the midfemur of children with QCP. All group differences in midfemur bone volume and width remained significant when femoral length was used as a covariate (p < 0.01).
Total width in the anterior–posterior and medial–lateral directions (a), width of the anterior, posterior, medial, and lateral aspects of the cortical wall (b), and medullary cavity width in the anterior–posterior and medial–lateral directions (c) of the middle-third of the femur in children with quadriplegic cerebral palsy (QCP) who are unable to ambulate independently and typically developing children. Values are means ± SE. *Group differences, p ≤ 0.001
Measures of bone distribution and estimates of midfemur strength are presented in Fig. 3. All measures were substantially lower in children with QCP. Specifically, CSMIap was 70% lower (d = 3.68, p < 0.001), CSMIml was 71% lower (d = 3.40, p < 0.001), Z ap was 61% lower (d = 3.81, p < 0.001), Z ml was 61% lower (d = 3.66, p < 0.001), and J was 71% lower (d = 3.69, p < 0.001) in the children with QCP. All group differences remained significant when femoral length was used as a covariate (p < 0.001).
Estimates of anterior–posterior and medial–lateral cross-sectional moment of inertia (CSMIap and CSMIml, respectively) and section modulus (Z ap and Z ml, respectively), and polar moment of inertia (J) in the middle-third of the femur in children with quadriplegic cerebral palsy (QCP) who are unable to ambulate independently and typically developing children. Values are means ± SE. *Group differences, p < 0.001
Discussion
The primary finding from the present study was that children with QCP and unable to ambulate independently had a markedly underdeveloped geometric structure and strength in the midfemur relative to typically developing children. Specifically, the volume of the midfemur and its cortical and medullary components was less than half the volume observed in typically developing children. Furthermore, the total midfemoral shaft was ~28% thinner with a medullary cavity that was ~27% thinner and a cortical wall that was 28% to 43% thinner in the anterior, posterior, and lateral aspects. The limited volume and thickness of the femoral shaft and its compartments in children with QCP was accompanied by an estimated resistance to bending and torsion that was more than 60% lower in the children with QCP. All group differences remained when the shorter length of the femur in children with QCP was statistically controlled suggesting that the group differences were not simply due to poor linear growth. The thinness of the femoral shaft and its cortical wall coupled with the very low aBMD and BMC previously reported in children with QCP [3, 4] makes the midfemur of a child with QCP extremely susceptible to fracture.
To our knowledge, this is the first study to assess bone structure and strength in the midshaft of a long bone in children with QCP. The idea that children with QCP have small thin bones is supported by a study of children with hemiplegic cerebral palsy in which bone area assessed by dual-energy X-ray absorptiometry was lower in the affected extremities compared to the unaffected extremities [27]. In a more recent study using peripheral quantitative CT, Binkley et al. [28] reported a substantially smaller distal tibia with markedly thinner cortical walls in a group of children and young adults with cerebral palsy (mainly nonambulatory) vs. controls. The impaired bone structure was associated with a 64% lower estimate of bone strength in the children with cerebral palsy. In addition to adding to our understanding about bone development in children with extreme mobility limitations, the findings from the present study have important clinical significance. In children with conditions that limit their ability to ambulate, such as children with QCP, most factures occur in the femur [2].
Another novel feature of the present study is the evaluation of the midfemur’s composition and geometric structure in different planes. An interesting finding was the substantially thinner anterior, posterior and lateral aspects of the cortical wall in the children with QCP than controls but no group difference in the medial aspect. The reason for the better cortical wall growth in the medial portion is not known. It is possible that the medial aspect vs. other aspects of the cortical wall is less influenced by loading induced by weight bearing or muscle contraction. Interestingly, a preferential erosion of the posterior section of the midfemur has been reported in adults with complete spinal cord injury [29]. Additional studies are needed to determine the reason for the select underdevelopment of the anterior, posterior, and lateral aspects of the femoral shaft and the more sustained growth in the medial aspect.
Until recently, the study of extreme physical inactivity on the geometric structure and strength of weight-bearing bone in humans was limited primarily to adults with spinal cord injury [29, 30]. Cross-sectional comparisons of adults with and without spinal cord injury indicate ~25% to 30% lower cortical volume (or cross-sectional area) and ~25% to 50% thinner cortical walls, but no difference in the total volume or width of the mid and distal ends of the tibia and femur [29, 30]. The compromised geometry results in ~13% to 30% lower estimates of bone strength (i.e., CSMI, Z, J, and polar bone strength strain index), which indicates a lower resistance to bending and torsion. Because the total volume and width of the bones are not compromised, the low estimates of strength are due solely to the thin cortical walls caused by erosion on the endosteal surface and an expansion of the medullary cavity. In the present study, in addition to thin cortical walls, the inner diameter of the medullary cavity and its total volume were ~50% smaller in children with QCP. Furthermore, the outer diameter and total volume of the midfemur were also ~50% smaller. The combined effect of thin cortical walls and a small thin shaft resulted in estimates of bone strength that were ~60–70% lower in children with QCP, which is more substantial than the decrement observed in adults with spinal cord injury. This suggests that chronic immobility that is present at birth abates the overall growth of the shaft in weight-bearing bones by limiting endosteal and periosteal expansion. It is likely that the poor bone growth during childhood results in adult bones that are small, thin, and easily fractured. Therefore, it is necessary to identify treatments that can facilitate bone growth in children with severe cerebral palsy.
Studying the bone structure of children with QCP using MRI, or other imaging modalities, is difficult because of the common presence of severe muscle spasms that lead to uncontrolled body movements. In addition to the muscle spasms, children with moderate to severe cerebral palsy often have poor cognitive function which further complicates magnetic resonance image collection. The resulting motion artifact could distort the images and lead to erroneous image analysis [31]. To minimize subject movement during the MRI scans, we used a novel immobilization procedure and an imaging protocol that took less than 7 min. In some of the children with QCP, motion was detected in the initial scan, and a second scan was required to obtain successful images. The steps taken to immobilize the subjects and to minimize scan time enabled collection of magnetic resonance images from children that typically require sedation. Images were successfully collected in ten of the 12 children with QCP who enrolled in the study suggesting that the approach has great promise as a method for assessing cortical bone structure in children with cerebral palsy. A similar approach was used to assess trabecular bone microarchitecture in children with QCP [4].
There are limitations to the present study that should be noted. Although it is inferred that the poor bone structure and strength estimates observed in the children with QCP are due to the lack of independent ambulation and the extremely low physical activity, other unknown factors related to the brain injury that causes cerebral palsy could have contributed to the poor bone development. Some anti-seizure medications, which are often taken by children with QCP, have been shown to impair bone metabolism [32]. However, in the present study, only two of the children with QCP were taking anti-seizure medications, and their measures of bone structure and strength were within the range of values in the group. There is also evidence that many children with QCP are undernourished [33, 34], which may contribute to their poor bone development. However, energy intake was not different in children with QCP and controls in the present study suggesting that the children with QCP were not undernourished. Although calcium intake in children with QCP was lower then the Dietary Recommended Intake for 9- to 18-year-old children [35], it was not different from calcium intake in controls. Some children with QCP who enrolled in the study were not able to complete testing because of fear of the MRI scanner (i.e., noise and/or claustrophobia). This indicates that MRI can not be used to assess bone structure and strength in all nonsedated children with severe forms of cerebral palsy. Despite this limitation, most children (ten or 12) were able to complete the tests successfully. Of the children who completed testing, some motion was detected in the image set, and a second scan was required to acquire images of good quality.
Conclusions
The present study indicates that the midfemur and its cortical wall and medullary cavity are much thinner and have much less volume in children with QCP who are unable to ambulate independently than typically developing children. The remarkable structural compromise contributes to very low estimates of bone strength in children with QCP. The present study also demonstrates the potential usefulness of MRI in the assessment of bone development in children with cerebral palsy and typically developing children. Future studies are needed to determine if the structural compromise in the midfemur of children with QCP can be improved with a mechanical loading, nutrition, or pharmacologic intervention.
References
McIvor WC, Samilson RL (1966) Fractures in patients with cerebral palsy. J Bone Joint Surg Am 48:858–866
Presedo A, Dabney KW, Miller F (2007) Fractures in patients with cerebral palsy. J Pediatr Orthop 27:147–153
Henderson RC, Lark RK, Gurka MJ, Worley G, Fung EB, Conaway M, Stallings VA, Stevenson RD (2002) Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics 110:e5
Modlesky CM, Subramanian P, Miller F (2008) Underdeveloped trabecular bone microarchitecture is detected in children with cerebral palsy using high-resolution magnetic resonance imaging. Osteoporos Int 19:169–176
Ciarelli TE, Fyhrie DP, Schaffler MB, Goldstein SA (2000) Variations in three-dimensional cancellous bone architecture of the proximal femur in female hip fractures and in controls. J Bone Miner Res 15:32–40
Kleerekoper M, Villanueva AR, Stanciu J, Rao DS, Parfitt AM (1985) The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif Tissue Int 37:594–597
Majumdar S, Link TM, Augat P, Lin JC, Newitt D, Lane NE, Genant HK (1999) Trabecular bone architecture in the distal radius using magnetic resonance imaging in subjects with fractures of the proximal femur. Osteoporos Int 10:231–239
Ott SM (1993) When bone mass fails to predict bone failure. Calcif Tissue Int 53(Suppl 1):S7–S13
Ferretti JL, Capozza RF, Zanchetta JR (1996) Mechanical validation of a tomographic (pQCT) index for noninvasive estimation of rat femur bending strength. Bone 18:97–102
Dempster DW (2000) The contribution of trabecular architecture to cancellous bone quality [editorial]. J Bone Miner Res 15:20–23
Link TM, Majumdar S, Augat P, Lin JC, Newitt D, Lu Y, Lane NE, Genant HK (1998) In vivo high resolution MRI of the calcaneus: differences in trabecular structure in osteoporosis patients. J Bone Miner Res 13:1175–1182
Parfitt AM (1987) Trabecular bone architecture in the pathogenesis and prevention of fracture. Am J Med 82:68–72
Siffert RS, Luo GM, Cowin SC, Kaufman JJ (1996) Dynamic relationships of trabecular bone density, architecture, and strength in a computational model of osteopenia. Bone 18:197–206
Woodhead HJ, Kemp AF, Blimkie CJR, Briody JN, Duncan CS, Thompson M, Lam A, Howman-Giles R, Cowell CT (2001) Measurement of midfemoral shaft geometry: repeatability and accuracy using magnetic resonance imaging and dual-energy X-ray absorptiometry. J Bone Miner Res 16:2251–2259
Bouxsein ML, Myburgh KH, van der Meulen MCH, Lindenberger E, Marcus R (1994) Age-related differences in cross-sectional geometry of the forearm bones in healthy women. Calcif Tissue Int 54:113–118
Jee D, Gilsanz V, Wren TA (2007) Limitations of peripheral quantitative computed tomography metaphyseal bone density measurements. J Clin Endocrinol Metab 92:4248–4253
Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL (2002) 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11:1–190
Miller F, Koreska J (1992) Height measurement of patients with neuromuscular disease and contractures. Dev Med Child Neurol 34:55–60
Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL (2000) CDC growth charts: United States. Adv Data 314:1–27
Tanner J (1962) Growth and adolescence. Blackwell Scientific, Oxford
Wood E, Rosenbaum P (2000) The gross motor function classification system for cerebral palsy: a study of reliability and stability over time. Dev Med Child Neurol 42:292–296
Turner CH, Burr DB (2001) Experimental techniques for bone mechanics. In: Cowen SC (ed) Bone mechanics handbook. CRC, Boca Raton, pp 1–35
Puyau MR, Adolph AL, Vohra FA, Zakeri I, Butte NF (2004) Prediction of activity energy expenditure using accelerometers in children. Med Sci Sports Exerc 36:1625–1631
Weight Control Information Network (August 2006) Just enough for you. In National Institutes of Health (NIH) Publication No 03–5287
USDA Food and Nutrient Database for Dietary Studies, 1.0. In. Agricultural Research Service, Food Surveys Research Group, Beltsville, MD
Cohen J (1988) Statistical power for the behavioral sciences. Lawrence Erlbaum, Hillsdale, NJ
Lin PP, Henderson RC (1996) Bone mineralization in the affected extremities of children with spastic hemiplegia. Dev Med Child Neurol 38:782–786
Binkley T, Johnson J, Vogel L, Kecskemethy H, Henderson R, Specker B (2005) Bone measurements by peripheral quantitative computed tomography (pQCT) in children with cerebral palsy. J Pediatr 147:791–796
Modlesky CM, Slade JM, Bickel CS, Meyer RA, Dudley GA (2005) Deteriorated geometric structure and strength of the mid-femur in men with complete spinal cord injury. Bone 36:331–339
Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, Schiessl H (2004) Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone 34:869–880
Wehrli FW, Hwang SN, Ma J, Song HK, Ford JC, Haddad JG (1998) Cancellous bone volume and structure in the forearm: noninvasive assessment with MR microimaging and image processing. Radiology 206:347–357
Lingam S, Joester J (1994) Spontaneous fractures in children and adolescents with cerebral palsy. BMJ 309:265
Stallings VA, Cronk CE, Zemel BS, Charney EB (1995) Body composition in children with spastic quadriplegic cerebral palsy. J Pediatr 126:833–839
Samson-Fang LJ, Stevenson RD (2000) Identification of malnutrition in children with cerebral palsy: poor performance of weight-for-height centiles. Dev Med Child Neurol 42:162–168
Standing Committee on the Scientific Evaluation of Dietary References Intakes, Food and Nutrition Board, Institute of Medicine (1997) Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and flouride. In: Institute of Medicine. National Academy Press, Washington, DC
Acknowledgements
The study was supported by the National Institutes of Health (HD050530), the National Osteoporosis Foundation and the United Cerebral Palsy Research and Educational Foundation. We express our deepest gratitude to all research participants and their families. We also thank the staff in the MRI Suite at the AI duPont Hospital for Children.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Modlesky, C.M., Kanoff, S.A., Johnson, D.L. et al. Evaluation of the femoral midshaft in children with cerebral palsy using magnetic resonance imaging. Osteoporos Int 20, 609–615 (2009). https://doi.org/10.1007/s00198-008-0718-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-008-0718-8