Abstract
Summary
The effect of teriparatide (20 μg/day) on serum calcium was examined in postmenopausal women previously treated with alendronate or raloxifene. Women previously treated with alendronate or raloxifene who added teriparatide or switched to teriparatide did not have clinically meaningful increases in mean predose serum calcium.
Introduction
The effects of a 6-month treatment with teriparatide (20 μg/day; rhPTH(1–34), TPTD) on serum calcium (Ca) was examined in a prospective study of postmenopausal women previously treated with alendronate (70 mg/week or 10 mg/day [ALN] or raloxifene 60 mg/d [RLX]) for ≥18 months.
Methods
Women continued their usual ALN or RLX during a 2-month antiresorptive phase. Women previously treated with ALN were randomized to add TPTD (n = 52) or switch to TPTD (n = 50) and women previously treated with RLX were randomized to add TPTD (n = 47) or switch to TPTD (n = 49). All were to take at least 500 mg/day of elemental Ca and 400–800 IU/day of vitamin D.
Results
Predose mean serum Ca did not significantly change in groups adding TPTD to either RLX or ALN treatment. In patients who switched from RLX or ALN to TPTD, mean serum Ca increased by 0.05 mmol/L and 0.04 mmol/L respectively. Only 1 patient had the predefined calcium endpoint of serum calcium > 2.76 mmol/L (11 mg/dL) at more than one visit.
Conclusions
Women previously treated with ALN or RLX who added TPTD or switched to TPTD did not have clinically meaningful increases in mean predose serum Ca.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Teriparatide, rhPTH (1–34), is a skeletal anabolic drug that increases bone mass, improves bone structure and strength, and reverses the progression of osteoporosis by increasing new bone apposition resulting in matrix and mineral composition consistent with younger bone [1–6]. Teriparatide treatment also increases serum calcium after dosing. Serum calcium peaks at about 4–6 h after injection and in general returns to normal prior to the next dose. In the Fracture Prevention Trial [7], a large study of postmenopausal women with osteoporosis, 11% of teriparatide- (20 μg/day) versus 2% of placebo-treated patients had at least one serum calcium value >2.65 mmol/L (10.6 mg/dL), from blood samples drawn 4–6 h after study drug injection. These elevations were not associated with adverse events, and serum calcium measurements collected more than 16 h post-dose were elevated in only 1 participant each from the teriparatide (20 μg/day) and placebo groups [8]. Many women with osteoporosis are currently being treated with antiresorptive drugs, including the selective estrogen receptor modulator raloxifene, and the bisphosphonate alendronate. In some women with severe osteoporosis who are currently taking such agents, anabolic treatment with teriparatide may become a clinical consideration. During the first 6 months of a study of women previously treated for 18–39 months with raloxifene (n = 26) or alendronate (n = 33) [9], mean serum calcium >12 h after teriparatide (20 μg/day) injection was increased from baseline after 1–6 months of teriparatide treatment by 0.09 to 0.14 mmol/L (0.36 to 0.56 mg/dL) [10]. Of the women pretreated with raloxifene, 3 (11.5%) had at least one predose serum calcium value >2.76 mmol/L (11.0 mg/dL); of those pretreated with alendronate, 3 (9.1%) had one or more predose serum calcium value >2.76 mmol/L (11.0 mg/dL) [10]. The highest serum calcium value reported was 3.12 mmol/L (12.5 mg/dL) [10]. None of the women had symptoms or complications associated with hypercalcemia. These findings differed from those observed in the teriparatide (20 μg) groups of phase 3 studies in osteoporosis drug-naïve men and postmenopausal women with osteoporosis [7, 8, 11]. To further investigate these findings, the current study was designed with input from the United States Food and Drug Administration to investigate the calcium safety of teriparatide in patients previously treated with raloxifene or alendronate.
Data regarding the efficacy and safety of switching to teriparatide versus adding teriparatide in patients previously treated with alendronate or raloxifene are not available. As such, this study included patients switching to teriparatide (20 μg/day) or adding teriparatide (20 μg/day) to raloxifene or alendronate therapy. Patients were studied during a 2-month phase while they continued their usual raloxifene or alendronate and a 6-month teriparatide treatment phase. The primary objective of this study was to evaluate predose serum calcium during the antiresorptive phase, and during the subsequent 6 months after switching from antiresorptive to teriparatide (20 μg/day). Efficacy findings from this study will be published elsewhere. A 6-month primary endpoint for this trial was selected because the frequency of patients who develop hypercalcemia after the first 6 months of teriparatide treatment was low during the Fracture Prevention Trial [7].
Materials and methods
Study design and patients
This study was an open-label, multi-center trial conducted at 11 study centers in the United States and included 198 postmenopausal women with osteoporosis who had been previously treated for at least 18 months with alendronate (any regimen providing the equivalent of a total of 70 mg/week) or raloxifene (60 mg/day). Patients entered the study into either a prior alendronate study stratum or a prior raloxifene study stratum based on their prior antiresorptive treatment. The study design consisted of two study phases, a 2-month antiresorptive phase and a 6-month teriparatide treatment phase. A 12-month extension of this study is currently ongoing (with different endpoints), but the results from the extension are not yet available.
Patients were included only if they were postmenopausal women, at least 50 years old, and had a previous clinical diagnosis of osteoporosis in the opinion of the investigator based on fracture history and/or bone density. Patients were required to have at least two vertebrae in the lumbar region (L1 through L4) that could be evaluated by dual X-ray absorptiometry (DXA) with Hologic (Hologic, Bedford, MA, USA) or Lunar (GE Medical Systems, Madison, WI, USA) densitometers and to have a current posterior–anterior lumbar spine bone mineral density (BMD) and/or hip BMD measurement more than 2.0 standard deviations below the average bone mass for young women (BMD T-score = −2.0). Patients had to have been taking stable calcium supplementation (at least 500 mg/day elemental calcium) for at least 1 month to enter the study. Patients were required to have serum calcium values within the normal range (8.9–10.1 mg/dL) as defined by a Mayo Clinic Medical Laboratory (Rochester, MN, USA) at the first visit of the antiresorptive phase. The reference range was determined using a population of volunteers who regularly donate blood for normal value studies. The adult age range is from 19 years and upward, with approximately equal numbers of men and women. Postmenopausal women were included. The reference range is checked every 3–4 years with 200+ normals each time, and has stayed constant since the mid-1960s. Patients were required to have normal or clinically insignificantly abnormal laboratory values (as determined by the investigator), including 25-hydroxyvitamin D (normal reference range 16–65 pg/mL), alkaline phosphatase, and thyroid stimulating hormone.
Patients were excluded if they had a history of hypercalcemia, with the exception of corrected hyperparathyroidism. They were also excluded if they had a history of: metabolic bone diseases other than osteoporosis, secondary causes of osteoporosis, or malignant neoplasms within the past 5 years. Additional exclusions included active urolithiasis within the past 2 years or high risk of urolithiasis in the opinion of the investigator, prior radiation therapy involving the skeleton, active liver disease (liver enzymes more than 3 times the upper limit of normal) or clinical jaundice, impaired renal function defined as serum creatinine >1.8 mg/dL, or history of excessive alcohol consumption. Additionally, patients were excluded if previously treated with drugs known to affect bone or calcium metabolism including: fluoride, teriparatide or any PTH analog at any time, bisphosphonates (excluding alendronate), androgens or other anabolic steroids, systemic corticosteroids with the past 12 months, estrogens, progestins, estrogen analogs, and agonists or any selective estrogen receptor analog other than raloxifene within the past 3 months. Finally, patients were excluded if treated in the prior 6 months with vitamin D >50,000 IU/week, or with any daily dose of 25-hydroxy- or 1,25-dihydroxyvitamin D, or with dihydrotachysterol.
Each patient provided written informed consent and institutional review board approval was obtained at each study center. All study methods and procedures were conducted in accordance with the ethical standards of the Declaration of Helsinki.
Treatments
All patients continued their usual alendronate or raloxifene treatments during the 2-month antiresorptive phase. At the end of this phase, patients were randomized (1:1) by a computer program to either continue or discontinue their usual alendronate (10 mg/day or 70 mg/week) or raloxifene (60 mg/day) treatments in unblinded fashion. All patients initiated teriparatide 20 μg/day by subcutaneous injection. Each patient’s vitamin D supplementation was adjusted to 400 to 800 IU/day at baseline. Patients were required to maintain their existing stable calcium regimen (at least 500 mg/day of elemental calcium) and vitamin D supplementation for the duration of the study. Patients provided their own calcium and vitamin D supplements and their own raloxifene or alendronate.
Baseline and follow-up assessments
Patients were asked to eat a similar meal before coming to each visit and to drink 6–8 oz of supplemental water in the morning prior to each visit to ensure adequate hydration. Blood draws were to be obtained approximately 24 h after the last teriparatide dose, and per protocol, at least 16 h from the last teriparatide dose [10]. Blood draws were performed at baseline, 1, and 2 months of the antiresorptive phase, and after 1, 3, and 6 months of the teriparatide treatment phase. Serum calcium was assessed by Mayo Clinic Medical Laboratories by measuring the complex formed between calcium and o-cresolphthalein, using the photometric absorbance at 600 nm (Roche Diagnostics, Indianapolis, IN, USA). Ionized calcium was assessed by local laboratories geographically close to the investigative sites. Twenty-four-hour urine collections were obtained 1 month prior to randomization and at 1, 3, and 6 months during the teriparatide phase. Additional assays included 25-hydroxyvitamin D (Diasorin™ Radio Immuno Assay; Covance, Princeton, NJ, USA) and 1,25-dihydroxyvitamin D (Diasorin™ Radio Immuno Assay; Covance), endogenous parathyroid hormone concentration (Diasorin™ Radio Immuno Assay; Covance), and bone-specific alkaline phosphatase (BSAP; Alkphase-B immunoassay; Pacific Biometrics, Seattle, WA, USA, interassay coefficient of variation, 5–8%). Per protocol, 24-h urinary excretion of calcium and creatinine were assessed by Covance Central Laboratories by measuring the complex formed between calcium and o-cresolphthalein in the presence of 8-hydroxyquinoline using photometry on a Roche Hitachi analyzer and normalized for a body surface area (BSA) of 1.73 m2 using the following equation [12]:
During the study, adverse events were collected at every visit, regardless of relationship to study medication. These events were captured as actual terms and coded to Medical Dictionary for Regulatory Affairs (MedDRA) terms by personnel blinded to treatment. Standard laboratory tests, including chemistry, hematology, and urinalysis panels, were also collected.
Definitions of hypercalcemia and hypercalciuria
Hypercalcemia substantial enough to raise concern about clinical adverse events required both elevation in serum calcium and sufficient duration to possibly cause adverse effects. Predose serum calcium values >2.76 mmol/L (11.0 mg/dL) were considered high enough to have possible clinical sequelae, and this level of calcium elevation was prospectively defined for analyses. In patients with primary hyperparathyroidism, long-term serum calcium levels >11.1 mg/dL have been associated with an increase in mortality, whereas serum calcium levels = 11.1 mg/dL do not appear to affect survival [13]. Sustained serum calcium >2.76 mmol/L was defined as at least two predose serum calcium measurements >2.76 mmol/L (11.0 mg/dL) at separate scheduled visits during a single phase of the study or discontinued treatment due to elevated serum calcium values. Confirmed serum calcium >2.76 mmol/L was defined as a serum calcium value both at a visit and a repeated measurement performed before the next visit. Similar analyses were conducted post-hoc using a predose serum calcium cut-off point of >2.60 mmol/L (10.4 mg/dL). The 2.60 mmol/L cut-off point was slightly higher than the upper limit of normal because it was considered that elevations minimally above the normal range likely have little clinical significance. In addition, although the protocol required the serum calcium value to be within the normal range at the first visit of the antiresorptive phase, in 22 out of 198 patients (11.1%, range 2.54 to 2.87 mmol/L) the first serum calcium measurement was above the normal range during the antiresorptive phase (8 at visit 1 and 7 each at visits 2 and 3) suggesting that mild slight elevations in serum calcium above the Mayo Clinic normal range were surprisingly common in this particular study population prior to initiation of teriparatide. The selected cut-off point was also qualitatively similar to the definition of hypercalcemia reported by Antoniucci et al. (upper limit of normal 2.57 mmol/L, hypercalcemia defined as serum calcium >2.62 mmol/L) and Greenspan et al. (upper limit of normal 2.55 mmol/L, hypercalcemia defined as serum calcium >2.66 mmol/L) [14, 15]. A definition of hypercalciuria was not provided in the protocol. Post-hoc analyses were conducted to assess the incidence of 24-h urinary calcium excretion above the upper limit of normal of 7.5 mmol/day (300 mg/day). Additional analyses were conducted to assess the incidence of 24-h urinary calcium excretion >10 mmol/day (400 mg/day).
Statistical analysis
For baseline characteristics, an ANOVA model was used to detect the difference between strata or the difference between treatment groups within a stratum for the continuous variables except that BSAP was analyzed using the Wilcoxon rank sum test. A study center-stratified Cochran-Mantel-Haenszel test was used for the categorical variables. Within-group and between-group comparisons of changes from the antiresorptive phase to each time point in the treatment phase were analyzed using a mixed model with repeated measures (MMRM) analysis. The MMRM model included fixed effects of treatment, visit, treatment-by-visit interaction, pooled study center, and the fixed covariate of baseline value, and was fitted using a restricted maximum likelihood method. The change from the mean of the antiresorptive phase to the mean of the treatment phase was analyzed using an ANCOVA model with treatment, study center, and baseline value included as covariates in the model. The proportion of patients with serum calcium values of >2.60 mmol/L and >2.76 mmol/L was compared between treatment groups using Fisher’s exact test. The comparison of the proportion of patients with serum calcium values of >2.60 mmol/L and >2.76 mmol/L between baseline and each post-baseline time point within a treatment group was analyzed using the McNemar test. For analyses of safety data, Fisher’s exact test was used to detect the difference between treatment groups. Correlations between baseline serum calcium, body mass index, age, endogenous PTH, serum 25-hydroxyvitamin D, and 1,25-dihydroxyvitamin D with maximum serum calcium during teriparatide treatment were assessed using Pearson correlation analysis. All analyses were performed on a modified intent-to-treat population with available data using SAS software (SAS Institute, Cary, NC, USA) at a significance level of 0.05 with no adjustment made for multiplicity.
Results
Patient disposition
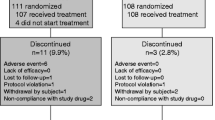
Patient disposition is shown in Fig. 1. Of 276 patients entered, 198 patients were enrolled into either a prior alendronate stratum (N = 102) or a prior raloxifene stratum (N = 96). Patients in the prior alendronate stratum were randomized to switch to teriparatide (n = 50) or to add teriparatide (n = 52). Similarly, patients in the prior raloxifene stratum were randomized to switch to teriparatide (n = 49) or to add teriparatide (n = 47). The number of patients who completed the study and the number of patients who withdrew from the study was not significantly different between treatment groups within the prior alendronate stratum (p = 0.27) or within the prior raloxifene stratum (p = 0.76).
Baseline characteristics
Baseline characteristics are presented in Table 1. In general, baseline characteristics were similar between strata and no significant differences were observed between treatment groups within either stratum. All but one patient was Caucasian. The median range of prior antiresorptive treatment was between 38 and 46 months among the various treatment groups (Table 1). The means of the baseline BSAP (range 11.6 to 29.6 U/L) were 20.0 and 20.1 U/L, in the groups previously treated with alendronate, and 25.1 and 27.1 U/L in the groups previously treated with raloxifene (between stratum p < 0.001). Patients previously treated with raloxifene had a slightly higher body mass index than those patients previously treated with alendronate (26.2 kg/m2 versus 24.0–24.5 kg/m2, p = 0.004).
Baseline serum calcium concentrations were higher in the prior alendronate versus raloxifene stratum; however, these differences were small and serum calcium corrected for albumin did not differ between the pretreatment groups. Twenty-four-hour urinary calcium excretion tended to be higher in the prior alendronate compared with the prior raloxifene stratum, but this difference was not statistically significant (p = 0.11; Table 1)
Predose serum calcium and hypercalcemia
Overall mean predose serum calcium significantly increased (p < 0.001) between the antiresorptive phase and the teriparatide phase by 0.042 mmol/L in patients who switched to teriparatide from alendronate and by 0.054 mmol/L in patients who switched to teriparatide from raloxifene (Fig. 2). Group mean predose serum calcium changes from baseline were significantly increased (p < 0.05) in the teriparatide phase at months 1, 3, and 6, in patients who switched to teriparatide from alendronate, by 0.028, 0.049, and 0.049 mmol/L respectively. Likewise, group mean predose serum calcium changes from baseline were significantly increased (p < 0.01) in the teriparatide phase at months 1, 3, and 6 in patients who switched to teriparatide from raloxifene, by 0.034, 0.063, and 0.075 mmol/L respectively (Fig. 2). In patients who added teriparatide to alendronate, change in mean predose serum calcium between the antiresorptive phase and the teriparatide phase and changes from baseline at months 1, 3, and 6 were not statistically significant (Fig. 2a). In patients who added teriparatide to raloxifene, the change in mean predose serum calcium between the antiresorptive phase and the teriparatide phase and changes from baseline at months 1 and 3 were not statistically significant (Fig. 2b). However, predose serum calcium at month 6 was statistically significantly increased by 0.034 mmol/L from baseline.
Predose serum calcium in patients previously treated with a alendronate (ALN) or b raloxifene who either switched to teriparatide (TPTD) or added teriparatide to antiresorptive treatment. Figure shows mean actual values ± SE. p < 0.05 for changes from baseline in patients who switched to teriparatide at all time points. Changes from baseline were not statistically significant in patients who added teriparatide. Baseline values represent the mean of the 2-month antiresorptive phase prior to adding or switching to teriparatide. Figure shows mean actual values ± SE. p < 0.01 for the change from baseline in patients who switched to teriparatide at all time points. p = 0.013 for the change from baseline at 6 months in patients who added teriparatide. Baseline values represent the mean of the 2-month antiresorptive phase prior to adding or switching to teriparatide
The incidence of serum calcium >2.60 mmol/L and of >2.76 mmol/L is shown in Tables 2 and 3. The incidence of elevated serum calcium was not statistically significant between treatment groups within either stratum. During the antiresorptive phase, 8 patients from the prior alendronate stratum had a serum calcium value of >2.60 mmol/L (8 out of 102, 7.8%) and 1 of these had a serum calcium value of >2.76 mmol/L (1 out of 102, 1.0%). During the antiresorptive phase, 1 patient from the prior raloxifene stratum had an elevated serum calcium value of >2.60 mmol/L and none of the patients had an elevated serum calcium value of >2.76 mmol/L.
During the teriparatide phase, 15 patients had a serum calcium value of >2.60 mmol/L (15 out of 198, 7.6%) with 11 having switched from antiresorptive to teriparatide and 4 having added teriparatide to antiresorptive treatment. Three patients (3 out of 198, 1.5%) had a serum calcium value of >2.76 mmol/L. Of these, 1 switched from raloxifene to teriparatide and 1 who added teriparatide to alendronate had normal ionized calcium values at the time their serum calcium value was >2.76 mmol/L, and did not have a serum calcium value of >2.76 mmol/L at any other assessment. Additionally, the patient who switched from raloxifene to teriparatide had her blood drawn 9.8 h post-dose rather than at least 16 h post-dose, as was specified in the protocol.
During the teriparatide phase, 2 patients (2 out of 198, 1.0%) had a confirmed serum calcium value of >2.60 mmol/L and 3 patients (3 out of 198, 1.5%) had a sustained serum calcium value of >2.60 mmol/L (10.4 mg/dL). Only 1 patient (1 out of 198, 0.5%) had a serum calcium value of >2.76 mmol/L (11.0 mg/dL), which was confirmed at retesting, and this patient also had a serum calcium value of >2.76 mmol/L at a subsequent visit. This patient was previously treated with alendronate and had serum calcium values of 2.6, 2.5, and 2.4 mmol/L during the antiresorptive phase, and, after switching to teriparatide, had serum calcium values of 2.8 and 2.9 mmol/L after 1 and 3 months of teriparatide. The serum calcium value of 2.9 mmol/L was the highest at a scheduled visit during the study, and she had a recheck serum calcium value of 3.2 mmol/L, which was the highest at any time during the study. Teriparatide therapy was discontinued, and, 3 months later, her serum calcium value was 2.4 mmol/L. The patient did not report any adverse side effects. The ratio of calcium clearance to creatinine clearance [Uca × Scr/Pca × Ucr] in this patient at baseline was 0.010, during teriparatide treatment it was 0.017 and 0.019, and after stopping teriparatide it was 0.008.
Predictors of serum calcium during the teriparatide treatment phase
Baseline serum calcium was significantly correlated (r = 0.51, p < 0.0001) with a maximum serum calcium value during teriparatide treatment. Baseline 25-hydroxyvitamin D was weakly correlated with a maximum serum calcium value (r = 0.15, p = 0.041). Other factors (body mass index, age, endogenous PTH, and 1,25-dihydroxyvitamin D) were not significantly correlated.
Twenty-four-hour urinary calcium excretion and hypercalciuria
Compared with baseline, 24-h urinary calcium excretion did not significantly increase after 1, 3, or 6 months of teriparatide treatment, with the exception of patients who switched to teriparatide from raloxifene at month 3 (p = 0.029). There were no statistically significant differences between treatment groups within each stratum for mean urinary calcium excretion (Fig. 3). The incidence of urinary calcium >7.5 mmol/day (300 mg/day) or of >10.0 mmol/day (400 mg/day) is shown in Table 4. The incidence of hypercalciuria was not statistically different from baseline at any time point within any treatment group except for at month 1 in patients who added teriparatide to raloxifene (p = 0.025). There were no statistically significant differences between treatment groups or between strata at any time point. However, the incidence of hypercalciuria using either definition was numerically higher at baseline and at all time points in patients previously treated with alendronate compared with patients previously treated with raloxifene (Table 4).
Twenty-four-hour urinary calcium excretion in patients previously treated with a alendronate or b raloxifene who either switched to teriparatide or added teriparatide to antiresorptive treatment. Values were not statistically different at any time point compared with baseline except at month 3 in patients who switched to teriparatide from raloxifene. Within each stratum, there was no significant difference between treatment groups at any time point. Patients were from the intent-to-treat population who had 24-h urinary calcium assessments during both the antiresorptive (baseline) and teriparatide treatment phases (months 1, 3, and 6). All values were normalized for a body surface area of 1.73 m2 [12]. Values are mean ± SE
Adverse events
Treatment-emergent adverse events (TEAEs) were similar between treatment groups in both the prior alendronate and prior raloxifene strata. The most frequently reported adverse events were arthralgia, dizziness, nausea, asthenia, headache, muscle spasms, and back pain. Hypercalcemia was reported as an adverse event in 2 patients, including 1 patient who switched to teriparatide from alendronate and 1 patient who added teriparatide to alendronate. In total, 10 patients (5.1%) discontinued the study due to an adverse event (Fig. 1). The frequencies of discontinuations due to an adverse event were not significantly different between treatment groups in either stratum. There were no adverse events that resulted in >1 discontinuation in any treatment group. No particular adverse events leading to hospitalization were reported by >1 patient with the exception of pneumonia, which was reported in 2 patients who switched to teriparatide from raloxifene. There were no deaths or reports of osteosarcoma.
Discussion
This is the first study randomizing postmenopausal women previously treated with alendronate or raloxifene to switch to teriparatide or to add teriparatide. Additionally, this is the first study specifically designed to assess the impact of teriparatide on serum calcium in patients previously treated with antiresorptive drugs. Compared with the current study, the study reported by Ettinger et al. [9] was smaller, conducted at single center, and was not specifically designed to examine calcium metabolism. In that study, all patients were switched overnight from prior alendronate or raloxifene to teriparatide. Serum calcium increased from baseline by 0.09–0.14 mmol/L (0.36–0.56 mg/dL), and intermittent, asymptomatic serum calcium >2.76 mmol/L (11 mg/dL) was observed in ∼10% of patients during the first 6 months of teriparatide treatment [9]. The calcium findings from that study do not agree with findings from other trials in teriparatide treatment-naïve patients [7, 8, 11]. This study was a multicenter trial specifically designed to examine the calcium response in patients who either switch to teriparatide or add teriparatide to antiresorptive treatment. Also, efforts were made in this trial to reduce variables known to influence serum calcium such as extended tourniquet time and inadequate patient hydration [16].
The physiological role of endogenous PTH is to control calcium homeostasis by regulating calcium reabsorption by the kidney and osteoclastic resorption in the bone, and indirectly, by regulating intestinal calcium absorption via 1,25-dihydroxyvitamin D. Teriparatide injected subcutaneously reaches a peak concentration after about 30 min and has a half life of approximately 1 h; concentrations are not quantifiable within 3 h of dosing [10]. Once-daily administration stimulates bone formation activity on trabecular and cortical bone surfaces by increasing the number and activity of osteoblasts without first stimulating bone resorption activity [17–23]. In contrast, continuous excess endogenous PTH as occurs in hyperparathyroidism is detrimental to the skeleton because bone resorption is stimulated more than bone formation [24–27]. This differential relative effect of intermittent and continuous exposure to PTH on bone resorption and bone formation likely results in less calcium efflux from the skeleton with subcutaneous teriparatide.
The observed lesser effect of teriparatide on serum calcium when added to ongoing antiresorptive drugs might be due to less bone resorption in these participants compared with those who switch to teriparatide. To support this hypothesis, data from another trial showed that teriparatide alone increased serum calcium while teriparatide plus raloxifene did not. The concomitant regimen also increased BMD significantly more at the total hip [28]. These results suggest the possibility that combination antiresorptive plus teriparatide may provide a more positive balance of calcium efflux into the skeleton.
Other potential mechanisms that may contribute to an increase in serum calcium with teriparatide use includes increased efficiency of calcium reabsorption in the distal nephron and the stimulation of 1,25-dihydroxyvitamin D production [7]. Indeed, 1,25-dihydroxyvitamin D has been implicated as a potential cause of hypercalcemia with use of teriparatide [29]. Although algorithms have been suggested to reduce calcium and vitamin D intake with elevated or high normal serum calcium levels while on teriparatide [29, 30], neither this study nor that of Ettinger et al. [9] included such adjustments. In addition, reduction in the teriparatide dose as was performed previously in treatment-naïve participants [7] was not employed in this trial. Since elevated serum calcium was uncommon with teriparatide approximately 24 h from its administration and in most cases not confirmed or sustained despite no change in the treatment regimen, the utility of such strategies may be of limited benefit. In addition, given the primary importance of the timing of serum calcium measurement in relation to teriparatide administration, routine serum calcium measurements in clinical practice while on teriparatide could potentially lead to undue concern and expense for patients.
Pre-existing hypercalcemia is an important consideration in patients with osteoporosis, in whom teriparatide therapy might be considered. Primary hyperparathyroidism is common in postmenopausal women with a peak incidence of 99 per 100,000 person years in 65- to 74-year-old women [13]. Several observations during this study highlight that abnormal serum calcium values may be observed in patients taking antiresorptive drugs. Of the 276 patients screened for this study, 15 patients (4.2%) were excluded by investigators for not having normal serum calcium at the first visit. Inspection of serum calcium in the 78 patients who failed screening revealed that 10 patients had serum calcium above the normal range and 7 patients had serum calcium below the normal range. Despite a requirement for normal serum calcium at the first visit, 9 of the 198 patients included in the study had serum calcium levels >2.60 mmol/L at antiresorptive phase visits prior to initiation of teriparatide. Patients with elevated serum calcium at baseline may experience elevations of serum calcium after initiation of teriparatide [10]. This point was illustrated in the current study by the average serum calcium during the antiresorptive phase being significantly correlated with maximum serum calcium during teriparatide treatment (r = 0.51, p < 0.001). The single patient with a sustained and confirmed serum calcium level of >2.76 mmol/L had a higher baseline serum calcium and lower urinary calcium excretion, endogenous PTH, and 1,25-dihydroxyvitamin D compared with other patients previously treated with alendronate and switched to teriparatide.
In conclusion, patient groups previously treated with alendronate or raloxifene who added teriparatide had no significant change in mean predose serum calcium and patient groups switched from prior alendronate or raloxifene to teriparatide had statistically significant, but not clinically meaningful increases in mean predose serum calcium. Overall, switching patients from antiresorptive treatment to teriparatide or adding teriparatide to antiresorptive treatment was safe and well tolerated in this population of postmenopausal women with osteoporosis.
References
Jiang Y, Zhao JJ, Mitlak BH et al (2003) Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res 18:1932–1941
Paschalis EP, Glass EV, Donley DW et al (2005) Bone mineral and collagen quality in iliac crest biopsies of patients given teriparatide: new results from the fracture prevention trial. J Clin Endocrinol Metab 90:4644–4649
Misof BM, Roschger P, Cosman F et al (2003) Effects of intermittent parathyroid hormone administration on bone mineralization density in iliac crest biopsies from patients with osteoporosis: a paired study before and after treatment. J Clin Endocrinol Metab 88:1150–1156
Ma YL, Zeng Q, Donley DW et al (2006) Teriparatide increases bone formation in modeling and remodeling osteons and enhances IGF-II immunoreactivity in postmenopausal women with osteoporosis. J Bone Miner Res 21:855–864
Lindsay R, Cosman F, Zhou H et al (2006) A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J Bone Miner Res 21:366–373
Keaveny TM, Donley DW, Hoffmann PF et al (2007) The effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosis. J Bone Miner Res 22(1):149–157
Neer RM, Arnaud CD, Zanchetta JR et al (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Krege JH, Donley DW, Marcus R (2005) Teriparatide, osteoporosis, calcium, and vitamin D. N Engl J Med 353:634–635
Ettinger B, San Martin J, Crans G et al (2004) Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 19:745–751
Forteo United States Package Insert (2004) Eli Lilly and Company
Orwoll E, Scheele WH, Paul S et al (2003) The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone mineral density in men with osteoporosis. J Bone Miner Res 18:9–17
Perez-Ruiz F, Calabozo M, Erauskin GG et al (2002) Renal underexcretion of uric acid is present in patients with apparent high urinary uric acid output. Arthritis Rheum 47:610–613
Wermers RA, Khosla S, Atkinson EJ et al (1998) Survival after the diagnosis of hyperparathyroidism: a population-based study. Am J Med 104:115–122
Antoniucci DM, Sellmeyer DE, Bilezikian JP et al (2007) Elevations in serum and urinary calcium with parathyroid hormone (1–84) with and without alendronate for osteoporosis. J Clin Endocrinol Metab 92:942–947
Greenspan SL, Bone HG, Ettinger MP et al (2007) Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med 146:326–339
Heath H III (2005) Postural and venous stasis-induced changes in total calcium. Mayo Clin Proc 80:1101
Wronski TJ, Yen CF, Qi H et al (1993) Parathyroid hormone is more effective than estrogen or bisphosphonates for restoration of lost bone mass in ovariectomized rats. Endocrinology 132:823–831
Hock JM (2001) Anabolic actions of PTH in the skeleton of animals. J Musculoskelet Neuronal Interact 2:33–47
Schmidt IU, Dobnig H, Turner RT (1995) Intermittent parathyroid hormone treatment increases osteoblast number, steady state messenger ribonucleic acid levels for osteocalcin, and bone formation in tibial metaphysis of hypophysectomized female rats. Endocrinology 136:5127–5134
Onyia JE, Bidwell J, Herring J et al (1995) In vivo, human parathyroid hormone fragment (hPTH 1–34) transiently stimulates immediate early response gene expression, but not proliferation, in trabecular bone cells of young rats. Bone 17:479–484
Dobnig H, Turner RT (1995) Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology 136:3632–3638
Shen V, Dempster DW, Birchman R et al (1993) Loss of cancellous bone mass and connectivity in ovariectomized rats can be restored by combined treatment with parathyroid hormone and estradiol. J Clin Invest 91:2479–2487
Whitfield JF, Morley P, Willick GE et al (1999) Stimulation of femoral trabecular bone growth in ovariectomized rats by human parathyroid hormone (hPTH)-(1–30)NH(2). Calcif Tissue Int 65:143–147
Heath D (1996) Familial hypocalcemia—not hypoparathyroidism. N Engl J Med 335:1144–1145
Dempster DW, Parisien M, Silverberg SJ et al (1999) On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism. J Clin Endocrinol Metab 84:1562–1566
Sato M, Miyauchi A, Takahara J (2000) Clinical aspects of hyperparathyroidism in Japanese multiple endocrine neoplasia type 1. Biomed Pharmacother 54 [Suppl 1]:86s–89s
Dobnig H, Turner RT (1997) The effects of programmed administration of human parathyroid hormone fragment (1–34) on bone histomorphometry and serum chemistry in rats. Endocrinology 138:4607–4612
Deal C, Omizo M, Schwartz EN et al (2005) Combination teriparatide and raloxifene therapy for postmenopausal osteoporosis: results from a 6-month double-blind placebo-controlled trial. J Bone Miner Res 20:1905–1911
Licata AA (2005) Osteoporosis, teriparatide, and dosing of calcium and vitamin D. N Engl J Med 352:1930–1931
Wermers RA, Khosla S, Atkinson EJ et al (2006) Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993–2001: An update on the changing epidemiology of the disease. J Bone Miner Res 21:171–177
Acknowledgements
We wish to thank Erik Eriksen, Sundeep Khosla, Robert Marcus, and Hunter Heath for their contributions to the design and initiation of the study. We thank David Donley for co-authoring the protocol, Rachel Wagman for assistance with implementing the study and Melinda Rance for assistance in the preparation of the figures.
Funding
Funding was provided by Lilly Research Laboratories, Indianapolis, Indiana, USA.
Conflicts of interest
Robert Wermers has received grant support from Eli Lilly and Company. Felicia Cosman has received consulting fees from Eli Lilly and Company. Li Xie, Emmett Glass, and John Krege are all employees of Eli Lilly and Company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wermers, R.A., Recknor, C.P., Cosman, F. et al. Effects of teriparatide on serum calcium in postmenopausal women with osteoporosis previously treated with raloxifene or alendronate. Osteoporos Int 19, 1055–1065 (2008). https://doi.org/10.1007/s00198-007-0557-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-007-0557-z