Abstract
Summary
Most studies of bone density in HIV-infected individuals focus on young men. This study compares differences in bone density in elderly HIV positive men and women to HIV negative controls. Bone density was lower in the lumbar spine and hip in the HIV-infected group. Antiretrovirals may be associated with decreased bone mineralization.
Introduction
Individuals with human immunodeficiency virus (HIV) may be at increased risk for osteoporosis. Prolonged exposures to HIV and/or antiretroviral therapy are possible causes for this association. This study compares differences in bone mineral density (BMD) in elderly HIV positive men and women to HIV negative controls.
Methods
A cross-sectional study was conducted among 57 HIV-infected and 47 HIV negative subjects over age 55. BMD at the lumbar spine and total hip and markers of bone turnover were compared.
Results
BMD was borderline lower in the lumbar spine and significantly lower in the hip in the HIV-infected group. Controlling for age, sex, race and body mass index, differences between the groups were significant at both sites. There was no difference in markers of bone turnover between the groups. Tenofovir use was significantly associated with decreased BMD at the spine while protease inhibitor use was significantly associated with decreased BMD at the hip.
Conclusion
Elderly men and women with HIV have lower bone mass than HIV negative controls. Decreased body mass index was the most important risk factor associated with decreased BMD. Bone demineralization was observed among HIV-infected subjects receiving either tenofovir or a protease inhibitor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the advent of highly active antiretroviral therapy (HAART) and the resultant decline in mortality, the life expectancy of HIV-infected individuals has increased [1]. Between 2001 and 2004, the percentage of all HIV cases in patients ≥50 years increased from 17% to 23% [2]. Although reports of decreased bone mineral density (BMD) predate the widespread use of HAART [3, 4], there has been increasing concern regarding the long-term effects of antiretroviral (ARV) therapy on BMD.
The prevalence of osteoporosis in HIV-infected individuals was recently reported to be 15%, or threefold higher than the general population [5]. Classical risk factors, such as hypogonadism and low weight, which may be increased in HIV-infected individuals, have been implicated as potential causes of decreased BMD as well as HIV infection and antiretroviral therapy.
The pro-inflammatory cytokines TNF-α, IL-1 and IL-6, which are induced by HIV infection, have been shown to increase osteoclastogenesis via increased production of receptor activator of nuclear factor κ B ligand (RANKL), the primary mediator of osteoclastogenic bone resorption [6–8]. Antiretrovirals have been shown to have an adverse effect on BMD [9–11]. Cross-sectional studies revealed a greater than twofold increase in osteoporosis in individuals treated with ARV therapy compared to ARV naïve patients with an increased odds ratio of 1.6 for individuals who received protease inhibitors compared to those who did not [5]. However, many of these studies did not control for other potential confounding variables. Specific antiretrovirals may have a significantly different impact on BMD [10, 12]. Tenofovir has been shown to decrease bone mineralization in children [12]. AZT has been shown to effect osteoclastogenesis and may influence BMD by a different mechanism [13].
In a study of older, post-menopausal women, time since menopause and weight were important predictors of BMD, whereas HIV related risk factors were not [14]. Importantly, this study was limited by the use of historical controls. The objective of this cross-sectional observational study was to compare the prevalence of decreased bone mineralization and associated risk factors in a cohort of older men and post-menopausal women to an HIV uninfected control population.
Methods
All HIV-infected patients over the age of 55 who attend the Jack Martin Fund Clinic, a New York State designated AIDS Center located at the Mount Sinai School of Medicine (MSSM), were offered enrollment in the study. In addition, five HIV-infected individuals were referred by community providers. Control subjects were recruited from the general internal medicine practice at MSSM and from providers in the surrounding community. Participants were excluded if they had hyperparathyroidism, hypercalcemia, Cushing’s disease, Paget’s or other known bone disease, or suppressed thyroid stimulating hormone (TSH) (<0.3 uIU/ml). Individuals who had received systemic corticosteroids for more than 6 months or other medications known to affect bone metabolism, such as bisphosphonates, were also excluded. Subjects were enrolled between May 2003 and March 2006. All individuals provided written informed consent and the study was approved by the Institutional Review Board (IRB) at MSSM, which conforms to the Helsinki Guidelines.
Participant evaluation included an interview and medical chart review to obtain duration of menopause, fracture history, detailed medication history (including type and duration of antiretroviral therapy), estrogen use, dietary calcium intake, extent of physical activity, alcohol consumption, illicit drug (heroin, cocaine) and tobacco use. Calcium intake was calculated by a Research Nutritionist using 24 hour dietary recall. Physical activity was defined as moderate or strenuous activity for at least 20 minutes on one or more days per week. Body mass index (BMI) and waist circumference were obtained and blood was collected for 25 hydroxyvitamin D and 1,25 dihydroxyvitamin D, intact parathyroid hormone (iPTH), thyroid stimulating hormone (TSH), luteinizing hormone (LH), follicular stimulating hormone (FSH), testosterone, estradiol, N-telopeptide, and osteocalcin. All control subjects underwent HIV testing by ELISA.
Bone mineral density (BMD) of the lumbar spine (L1-4) and total hip were measured by dual-energy x-ray absorptiometry using a Hologic 4500 densitometer (Hologic Inc., Waltham, MA). Control data used by this machine are derived from the Second National Health and Nutrition Examination Survey (NHANES II).
Statistical analysis
Descriptive statistics were used to characterize the groups. Chi-square or Fisher’s exact test was used for comparison of categorical variables between the two groups. A non-parametric Wilcoxon test was performed to compare cases and controls for all continuous variables other than age, weight, BMI which were compared by Student’s t-test. For the sex hormones (LH, FSH, testosterone and estradiol) non-parametric tests were performed independently for males and females using a p value of <.025 for significance. Multivariate analysis was used to test for differences between HIV positive and HIV negative participants in bone mineral density, adjusting for age, sex, race and BMI. Within the HIV positive group, multiple regression analysis was used to determine if use and duration of specific antiretroviral agents (tenofovir and protease inhibitors) were related to bone mineral density, when controlling for age, gender, BMI, and duration of HIV infection. In order to retain all participants in this analysis, one missing value of tenofovir duration, three missing values of PI duration and a single missing value of HIV duration were replaced by the mean. Calculations were done using SAS software (version 9; SAS Institute). All tests were two-tailed.
Results
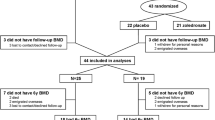
A total of 115 subjects were enrolled. Eleven were excluded for the following reasons: four for hyperparathyroidism, two for corticosteroid use of more than 6 months, three individuals did not return for DEXA scanning and two were unable to have DEXA scans performed. Of the remaining 104 study participants, 57 were HIV-infected individuals and 47 were HIV negative controls. Among the HIV-infected subjects, 82% were receiving antiretroviral therapy and 52% of the total were on protease inhibitors. Characteristics of HIV-infected and control subjects are shown in Tables 1, 2 and 3. The mean age and median years since menopause were similar between the cases and controls. However there were significantly more men in the HIV positive group, as well as more African Americans. Body mass index (BMI) and weight were significantly lower in the HIV positive group.
HIV-infected individuals were more likely to be smokers than controls. A history of cocaine or heroin use was significantly higher among HIV-infected subjects than controls. The HIV-infected group was less likely to be physically active. Among the HIV-infected subjects, only 16% reported current alcohol consumption compared to 49% of controls. Vitamin D levels were noted to be low; however there was no difference in levels between HIV-infected individuals and control subjects. Furthermore, there was no difference in iPTH, TSH, fracture history, estrogen use, steroid use, calcium intake or markers of bone turnover (osteocalcin and N-telopeptide) (Tables 1 and 2). There was a significant difference in LH between HIV-infected women compared to HIV negative women (p = .01). FSH, testosterone and estradiol did not significantly differ between HIV-infected and control subjects (data not shown).
Table 4 gives descriptive statistics for bone density measurements (T scores and Z scores) for both groups. All measures of bone density were significantly lower in the HIV positive individuals. Table 5 shows that controlling for age, sex, race and BMI, differences between the groups were generally significant at both sites. Differences in the hip were highly significant (p < .01), while for the spine, differences were significant for the Z score (p < .05), and borderline significant for the T score (p = .072).
No association was found between degree of bone demineralization and current or nadir CD4 cell count, duration of HIV infection, or viral load (data not shown). After adjusting for age, gender, BMI, duration of HIV and PI use, duration of tenofovir use was significantly associated with decreased T and Z score at the spine (p = 0.007, 0.007, respectively) but not at the hip (Table 6). In contrast, after adjusting for the above variables and tenofovir rather than duration of PI use, duration of protease inhibitor use was significantly associated with decreased T score at the hip (p = 0.017), but not at the spine. The difference in Z score at the hip was of borderline significance (p = 0.054).
In this study, the prevalence of abnormal bone density (osteopenia + osteoporosis) is significantly increased in HIV positive individuals compared to HIV negative controls (Table 7). In both groups, osteoporosis which is defined as a T score >2.5 standard deviations below peak bone mass, was more common at the lumbar spine rather than the hip.
Discussion
Most studies of BMD and HIV infection have focused on men or have lacked a comparison group of individuals without HIV infection. This cross-sectional study examined the prevalence of decreased bone mineral density in one of the oldest groups of HIV-infected individuals studied thus far. We found that elderly HIV-infected men and women have a significantly higher prevalence of decreased BMD when compared to an HIV negative control population. In a study by Arnsten et al., femoral neck and lumbar spine BMD were reduced in women with HIV infection, compared with women without HIV infection [15]. However, the median age of the women was 44 years compared to this study in which the mean age of subjects was 61. In a recently published study, femoral neck and lumbar spine BMD were significantly lower in ≥49 year old HIV-infected compared with HIV-uninfected men [16].
In this study, classical risk factors for osteoporosis such as BMI were more important contributors to decreased BMD in HIV-infected individuals than HIV related risk factors, which have been previously reported to be associated with decreased BMD, such as duration of HIV infection, current or nadir CD4 cell count or viral load. An exception to this finding was the use of tenofovir or protease inhibitors. Tenofovir has been shown in longitudinal studies to be associated with decreased bone mineralization [10, 12], potentially due to renal phosphate wasting and subsequent osteomalacia [17]. In this study, the longer subjects received tenofovir, the lower the BMD at the spine and the longer participants took PIs the lower the BMD at the hip. Notably, the effect of tenofovir at the hip exceeds that for PI even though only the latter is significant (Table 6). Presumably, this is due to the wider range and longer duration of PI use.
The spine is comprised primarily of cancellous bone, which undergoes more rapid turnover than the hip, which consists mostly of cortical bone [18]. The differential effect seen by tenofovir and PI at these two sites may, therefore, reflect different mechanisms by which BMD may be effected. Further study to confirm these findings and elucidate the possible mechanisms is needed. In addition, combined use of both tenofovir and protease inhibitors may result in an additive effect on BMD and warrants further study.
Although LH levels were higher in HIV-infected than HIV negative women, there was no difference in estradiol levels. Therefore, the significance of this finding is unclear. Both study groups had low 25 hydroxyvitamin D levels. However this is not an unexpected finding for a low income, urban population living in the northern hemisphere and has been previously reported [19]. Protease inhibitors have been implicated in suppressing the synthesis of 1,25 dihydroxyvitamin D [20]. Other authors have found lower levels of 1,25 dihydroxyvitamin D in HIV-infected individuals regardless of protease inhibitor use [21, 22]. TNF-α inhibition of 1α hydroxylase activity has been suggested as a possible mechanism for this finding. However, we did not see a difference in 1,25 dihydroxyvitamin D levels between HIV-infected individuals and controls. Although serum calcium levels were higher in the HIV negative group, ionized calcium was not obtained. Thus, it is unclear if the biologically relevant form of calcium was different between the groups.
Aukrust et al. described perturbations in markers of bone metabolism in HIV- infected individuals with advanced disease. Specifically, low osteocalcin, a marker for bone formation, and high C-telopeptide, a marker for bone resorption, were seen. Notably after institution of HAART, a marked increase was seen in osteocalcin levels coincident with a decrease in HIV viral load and levels of TNF-α [23]. Furthermore, post HAART, a shift towards resynchronization of bone remodeling was seen as evidenced by a greater correlation between osteocalcin and C-telopeptide levels. Dolan et al. found an increase in N-telopeptide in HIV-infected individuals compared with control subjects and that there was an inverse correlation between BMD and urinary N-telopeptide level [24]. However, in a study that looked only at HIV-infected individuals, no association between BMD and bone markers was seen [25]. We did not find any difference in osteocalcin and N-telopeptide levels between the two groups. Specifically, there was no evidence of uncoupling between osteocalcin and N-telopeptide in the HIV positive group as has been previously noted [23]. However, this is not unexpected as >80% of subjects were on antiretroviral therapy. Osteocalcin and N-telopeptide were not collected fasting and in the morning in all subjects, which is a limitation of the study.
An additional limitation of this study is the small sample size, which limited the ability to test for interactions between covariates. Of note, there were significant differences between the groups with respect to alcohol consumption, tobacco use and physical activity which are factors known to influence bone mineral density. However, the small number of participants limited the number of variables the model could accommodate.
To minimize the possibility of referral bias, the vast majority of subjects were recruited from a single clinic and all patients in the clinic were approached and offered participation in the study. Although there is the potential for referral bias in the control population, this would have skewed results towards a higher prevalence of decreased BMD in the control group, which is not what was found. Although there was more illicit drug use in the HIV-infected compared to the HIV- uninfected group (15 vs. 2 reported ever using heroin and 19 vs. 1 reported ever using cocaine), the study was limited by the absence of data on current drug use.
There was no statistically significant difference between the groups with respect to systemic steroid use; however, it should be noted that we did not collect data on the use of inhaled or topical steroids. Given the increasing awareness of the potential for systemic effects from inhaled and topical steroids due to pharmacokinetic interactions with protease inhibitors, this may have been a significant omission [26].
This study supports the need to be vigilant in screening and early intervention for both osteopenia and osteoporosis in HIV-infected individuals as they age, especially those with underlying risk factors. It also underscores the need to be mindful of the potential contribution of underlying vitamin D deficiency, particularly in an urban poor population. The above is especially true given the prolonged period that HIV-infected individuals may remain on HAART. The potential long-term effect of tenofovir and protease inhibitors deserves particular attention and longitudinal studies investigating this are ongoing.
References
Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD (1998) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338:853–860
Gebo KA (2006) HIV and aging: implications for patient management. Drugs Aging 23:897–913
Paton NI, Macallan DC, Griffin GE, Pazianas M (1997) Bone mineral density in patients with human immunodeficiency virus infection. Calcif Tissue Int 61:30–32
Serrano S, Marinoso ML, Soriano JC, Rubies-Prat J, Aubia J, Coll J, Bosch J, Del Rio L, Vila J, Goday A et al (1995) Bone remodelling in human immunodeficiency virus-1-infected patients. A histomorphometric study. Bone 16:185–191
Brown TT, Qaqish RB (2006) Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. Aids 20:2165–2174
Fakruddin JM, Laurence J (2004) Interactions among human immunodeficiency virus (HIV)-1, interferon-gamma and receptor of activated NF-kappa B ligand (RANKL): implications for HIV pathogenesis. Clin Exp Immunol 137:538–545
Armstrong AP, Tometsko ME, Glaccum M, Sutherland CL, Cosman D, Dougall WC (2002) A RANK/TRAF6-dependent signal transduction pathway is essential for osteoclast cytoskeletal organization and resorptive function. J Biol Chem 277:44347–44356
Manolagas SC, Jilka RL (1995) Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med 332:305–311
Tebas P, Powderly WG, Claxton S, Marin D, Tantisiriwat W, Teitelbaum SL, Yarasheski KE (2000) Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. Aids 14:F63–F67
Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, Coakley DF, Lu B, Toole JJ, Cheng AK (2004) Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. Jama 292:191–201
Carr A, Miller J, Eisman JA, Cooper DA (2001) Osteopenia in HIV-infected men: association with asymptomatic lactic acidemia and lower weight pre-antiretroviral therapy. Aids 15:703–709
Hazra R, Gafni RI, Maldarelli F, Balis FM, Tullio AN, DeCarlo E, Worrell CJ, Steinberg SM, Flaherty J, Yale K, Kearney BP, Zeichner SL (2005) Tenofovir disoproxil fumarate and an optimized background regimen of antiretroviral agents as salvage therapy for pediatric HIV infection. Pediatrics 116:e846–e854
Pan G, Yang Z, Ballinger SW, McDonald JM (2006) Pathogenesis of osteopenia/osteoporosis induced by highly active anti-retroviral therapy for AIDS. Ann N Y Acad Sci 1068:297–308
Yin M, Dobkin J, Brudney K, Becker C, Zadel JL, Manandhar M, Addesso V, Shane E (2005) Bone mass and mineral metabolism in HIV+ postmenopausal women. Osteoporos Int 16:1345–1352
Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Santoro N, Schoenbaum EE (2006) HIV infection and bone mineral density in middle-aged women. Clin Infect Dis 42:1014–1020
Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Lo Y, Klein RS (2007) Decreased bone mineral density and increased fracture risk in aging men with or at risk for HIV infection. Aids 21:617–623
Peyriere H, Reynes J, Rouanet I, Daniel N, de Boever CM, Mauboussin JM, Leray H, Moachon L, Vincent D, Salmon-Ceron D (2004) Renal tubular dysfunction associated with tenofovir therapy: report of 7 cases. J Acquir Immune Defic Syndr 35:269–273
Ichiba H, Hirai C, Fujimaru M, Shintaku H, Yamano T, Funato M (2001) Measurement of bone mineral density of lumbar spine and whole body in low-birth-weight infants: comparison of two methods. J Bone Miner Metab 19:52–55
Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B (2000) Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab 85:4125–4130
Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS (2003) HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. Aids 17:513–520
Haug CJ, Aukrust P, Haug E, Morkrid L, Muller F, Froland SS (1998) Severe deficiency of 1,25-dihydroxyvitamin D3 in human immunodeficiency virus infection: association with immunological hyperactivity and only minor changes in calcium homeostasis. J Clin Endocrinol Metab 83:3832–3838
Madeddu G, Spanu A, Solinas P, Calia GM, Lovigu C, Chessa F, Mannazzu M, Falchi A, Mura MS, Madeddu G (2004) Bone mass loss and vitamin D metabolism impairment in HIV patients receiving highly active antiretroviral therapy. Q J Nucl Med Mol Imaging 48:39–48
Aukrust P, Haug CJ, Ueland T, Lien E, Muller F, Espevik T, Bollerslev J, Froland SS (1999) Decreased bone formative and enhanced resorptive markers in human immunodeficiency virus infection: indication of normalization of the bone-remodeling process during highly active antiretroviral therapy. J Clin Endocrinol Metab 84:145–150
Dolan SE, Huang JS, Killilea KM, Sullivan MP, Aliabadi N, Grinspoon S (2004) Reduced bone density in HIV-infected women. Aids 18:475–483
Fausto A, Bongiovanni M, Cicconi P, Menicagli L, Ligabo EV, Melzi S, Bini T, Sardanelli F, Cornalba G, Monforte A (2006) Potential predictive factors of osteoporosis in HIV-positive subjects. Bone 38:893–897
Samaras K, Pett S, Gowers A, McMurchie M, Cooper DA (2005) Iatrogenic Cushing’s syndrome with osteoporosis and secondary adrenal failure in human immunodeficiency virus-infected patients receiving inhaled corticosteroids and ritonavir-boosted protease inhibitors: six cases. J Clin Endocrinol Metab 90:4394–4398
Acknowledgments
This work was supported by Grant Number M01-RR-00071 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jones, S., Restrepo, D., Kasowitz, A. et al. Risk factors for decreased bone density and effects of HIV on bone in the elderly. Osteoporos Int 19, 913–918 (2008). https://doi.org/10.1007/s00198-007-0524-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-007-0524-8