Abstract
Summary
The demographic and clinical characteristics of patients initiating teriparatide were compared with those of patients initiating bisphosphonates for the treatment of osteoporosis. In these samples of commercially insured, Medicare, and Medicaid patients, patients initiating teriparatide were older, in poorer health, and appeared to have more severe osteoporosis than patients initiating bisphosphonates.
Introduction
The demographic and clinical characteristics of patients initiating teriparatide are compared with those of patients initiating bisphosphonates.
Methods
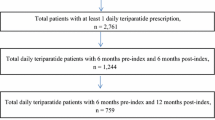
Beneficiaries (45 years and older) with at least one claim for teriparatide or a bisphosphonate from 2003 to 2005 and continuous enrollment in the previous 12 months and subsequent 6 months were identified from commercial, Medicare, and Medicaid administrative claims databases. Patients initiating teriparatide (commercial/Medicare (N = 2,218); Medicaid (N = 824)) were compared to patients initiating bisphosphonates (commercial/Medicare (N = 97,570); Medicaid (N = 77,526)) in terms of age, provider specialty, comorbidities, prior use of osteoporosis medications, fractures, BMD screening, health status, and resource utilization.
Results
Teriparatide patients were older and in poorer health than bisphosphonate patients. Approximately 38% of teriparatide patients in both groups had fractured in the pre-period compared to 16% of commercial/Medicare and 15% of Medicaid bisphosphonate patients. Teriparatide patients were more likely to have used osteoporosis medications in the pre-period (79.9% versus 32.1% (commercial/Medicare); 82.2% versus 19.6% (Medicaid)).
Conclusions
In these samples of patients, those initiating teriparatide differed from those initiating bisphosphonates. Teriparatide patients were older, in poorer health, and appeared to have more severe osteoporosis than bisphosphonate patients. Comparisons of treatment outcomes should take these differences in patient characteristics into consideration.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Currently, antiresorptive agents, including bisphosphonates, are considered first-line treatment for osteoporosis [1]. Bisphosphonates work primarily by reducing bone resorption and preserving bone mass. Teriparatide (recombinant parathyroid hormone 1–34), is a bone formation agent that has been shown to increase bone mineral density (BMD) and reduce the risk of fractures [2]. A self-administered daily injectable, it is indicated for the treatment of postmenopausal women with osteoporosis who are at a high risk for fracture or for women who have failed or are intolerant of previous osteoporosis therapy [3]. Teriparatide is also indicated for men with primary or hypogonadal osteoporosis who are at a high risk of fracture. Given the unique pharmacologic mechanism of action of teriparatide and its current parenteral formulation, it is important to understand for whom the treatment is prescribed and how these patients differ from those receiving treatment with the more commonly prescribed bisphosphonates. The primary objective of this study is to describe and compare the demographic and clinical characteristics of patients who initiate teriparatide therapy relative to patients initiating treatment with bisphosphonates.
Materials and methods
This study was conducted using a retrospective cohort design. Data are from the Thomson MarketScan® Research databases, which include the healthcare experience of 33 million individuals covered by a variety of private health plans, as well as Medicare and Medicaid. Individuals with Medicare coverage are Medicare-eligible retirees with employer sponsored Medicare supplemental plans. Service claims during the period January 2002 through June 2005 were assessed for the commercial and Medicare-insured populations. Because a relatively small number of patients initiated teriparatide overall, the commercial and Medicare data were combined. Due to limitations in data availability, service claims for Medicaid enrollees were examined between January 2003 and December 2004. Data sources contain the pooled healthcare experience of enrollees and include records of inpatient admissions, inpatient services, outpatient services, and prescription drug claims.
The study population consists of individuals ages 45 and older who initiated treatment with teriparatide (Forteo®, rhPTH(1–34), Eli Lilly) or a bisphosphonate (i.e., alendronate, etidronate, ibandronate, and risedronate) anytime during the period from January 2003 to December 2004 (June 2004 for Medicaid). The date of the first prescription claim for teriparatide or a bisphosphonate in this period represents the index date for each patient. Patients were required to have a pre-period of 365 days before and a post-period of at least 180 days following the index date. Because the focus of this study is on new users, teriparatide and bisphosphonate patients with a prescription for teriparatide or a bisphosphonate, respectively, during the pre-period were excluded from the study. Patients were excluded if they did not have prescription coverage in the pre- and post-periods or had an index prescription with a days supply of 0 or more than 180 days.
Measures
Demographic variables were defined as of the index date and included age, gender, race (for Medicaid patients only), regional location (for commercial/Medicare patients only), and urban/rural residence. The specialty of the provider most closely associated with the index prescription was identified by examining all claims for “evaluation and management” office visits in the 60 days prior to the index date. Claims with an osteoporosis diagnosis were scanned first to find the most temporally proximal claim to the prescription. If no claim with an osteoporosis diagnosis was found, then all claims in the 60-day period were scanned to identify the one closest to the prescription date. Provider specialty was coded as primary care (i.e., medical doctor, internist, family doctor, and osteopath), specialist (i.e., geriatric specialist, obstetrician/gynecologist, endocrinologist, nephrologist, rheumatologist, physical therapy or rehabilitation specialist, or orthopedic surgeon), or other.
Clinical characteristics included assessments of comorbid illness, prior fractures and BMD screening, prior and concomitant medication use, and prior resource utilization. Ascertainment of all historical data, including prior fracture, comorbidity and resource use, was confined to the 365-day period preceding the index prescription. The Charlson Comorbidity Index (CCI) and the Chronic Disease Score (CDS) were calculated as proxies for overall health status [4, 5]; other comorbidities associated with bone complications (e.g., cancer, Paget’s disease, and endocrine diseases) were examined separately. HEDIS definitions were used to identify prior fractures, further categorized as hip, vertebral, or other non-vertebral, and to identify patients who received a BMD assessment in the pre-period. Prior use of medications that can precipitate bone loss (e.g., hormone deprivation therapy and glucocorticoids) and prior or concomitant use of other osteoporosis treatments (e.g., raloxifene, calcitonin, and estrogen/hormone treatments) were also assessed. Finally, prior inpatient, emergency room, outpatient, and pharmaceutical claims were quantified.
Analysis
Patients initiating teriparatide or a bisphosphonate are compared in terms of demographic and clinical characteristics, as well as resource utilization. Statistical tests of significance for differences between groups were carried out as appropriate. Group comparisons were made using chi-square tests for categorical measures and Wilcoxon rank-sum tests for continuous variables.
Results
A total of 360,162 commercial/Medicare and 185,168 Medicaid patients received treatment with either a bisphosphonate or teriparatide in 2003–2004. Of these, fewer than 1% received teriparatide (4,017 commercial/Medicare and 975 Medicaid). After applying the inclusion/exclusion criteria, we had 2,218 commercial/Medicare and 824 Medicaid patients with teriparatide. In addition, 97,570 commercial/Medicare and 77,526 Medicaid patients with a bisphosphonate met the inclusion/exclusion criteria. Less than 1% of patients were excluded due to criteria related to the index prescription.
Demographic differences
On average, patients taking teriparatide were significantly older than were bisphosphonate patients (Table 1). Teriparatide patients in the Medicaid population were less likely to live in urban areas relative to bisphosphonate patients, and were more likely to be white than African American, Hispanic/Latino or Asian. Patients initiating teriparatide in both populations were more likely to have seen a specialist around the time of their index prescription.
Pre-period clinical characteristics
Patients initiating teriparatide were in poorer health than patients initiating bisphosphonate therapy in both groups, as indicated by their higher CCI and CDS scores (Table 2). For example, the mean CCI score of patients with teriparatide was approximately 38.0% higher in the commercially insured/Medicare group and 21.4% higher in the Medicaid group than patients with a bisphosphonate. Further, higher proportions of patients with teriparatide in both groups had rheumatoid arthritis and cardiovascular disease compared to patients initiating a bisphosphonate. Approximately 38% of patients with teriparatide in both groups had fractured within the past year. In contrast, 16% of commercially insured/Medicare and 15% of Medicaid bisphosphonate patients had a fracture in the pre-period.
About 62% of commercially insured/Medicare patients had BMD screening in the pre-period, irrespective of therapy type, compared to fewer than one-third of Medicaid patients. In both groups, a higher percentage of patients with teriparatide had a pre-period glucocorticoid prescription (29.9% teriparatide versus 17.7% bisphosphonates (commercially insured/Medicare); 32.2% teriparatide versus 17.1% bisphosphonates (Medicaid)). Teriparatide patients were also more likely to have concurrent or previous osteoporosis medication use than patients on bisphosphonates (79.9% versus 32.1% (commercially-insured/Medicare); 82.7% versus 19.6% (Medicaid)).
With regard to resource utilization, patients taking teriparatide had more pre-period claims of all types than did patients taking a bisphosphonate in both populations (Table 2). For example, the mean number of pre-period outpatient claims among patients with teriparatide was 37.4% and 34.7% higher than the means for patients with a bisphosphonate in the commercially insured/Medicare and Medicaid groups, respectively.
Discussion and conclusion
The results of this study suggest that the characteristics of patients who initiate teriparatide are different from those that initiate bisphosphonates. Teriparatide patients were older, more severely ill, and appeared to have more severe osteoporosis than patients using bisphosphonates. These findings are observed within both the commercially insured/Medicare and Medicaid populations.
Teriparatide is indicated for the treatment of postmenopausal women with severe osteoporosis, or for those who have not adequately benefited from or who are intolerant of other osteoporosis therapy. Among the relatively small number of patients initiating teriparatide in the present study, a significant proportion had a pre-period fracture and nearly 80% had received a previous (non-teriparatide) anti-osteoporotic medication.
A few limitations of the data should be noted to the present study. Standard limitations of claims data apply to this study [6]. Because these data rely on the coding decisions of a variety of professionals, the potential for coding errors or omissions in claims data should be recognized.
It should also be noted that the commercially insured cohort in this study was derived from a convenience sample of individuals with employer-sponsored private health insurance (MarketScan). Compared to the national population of individuals with employer sponsored insurance, MarketScan has a higher concentration of people in the South and lower in the Northeast. Additionally, the Medicaid data utilized in this study represent eight states only and thus are not likely to be representative of the entire Medicaid population initiating treatment for osteoporosis across the United States. Further, Medicare patients included in this study are those with supplemental insurance provided by employers. Because the characteristics and experiences of patients with Medicare coverage alone may differ from those with supplemental insurance, the extent to which our results are generalizable to the entire Medicare population is unknown.
Finally, the formulary status of the medications was unavailable. Therefore, it is unclear how formulary restrictions (e.g., prior authorization) may have impacted which patients received teriparatide.
Conclusion
The findings of this study suggest that patients initiating teriparatide for the treatment of osteoporosis differ from those who initiate bisphosphonate treatment. Patients initiating teriparatide therapy tend to be older and in poorer health than patients initiating bisphosphonate therapy. Comparisons of treatment outcomes associated with these medications should take these important differences in patient characteristics into consideration.
References
American College of Obstetrics and Gynecology (2004) Osteoporosis: Clinical management guidelines for the obstetrician-gynecologist. Obstet Gynecol 103:203–216
Neer RM, Arnaud, CD, Zanchetta, JR et al. (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Forteo package insert. (2002) Forteo (teriparatide) by Eli Lilly and Company. Accessed online January 18, 2007, at: http://pi.lilly.com/us/forteo-pi.pdf
Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–619
Von Korff M, Wagner EH, Saunders K (1992) A chronic disease score from automated pharmacy data. J Clin Epidemiol 45:197–203
Birnbaum HG, Cremieux PY, Greenberg PG et al. (1999) Using healthcare claims data for outcomes research and pharmacoeconomic analysis. Pharmacoeconomics 16:1–8
Acknowledgements
We gratefully acknowledge the assistance of Emily Durden in the preparation of this manuscript. Funding for this analysis was provided by Eli Lilly and Company.
Disclosure
Shonda Foster, Eric Meadows, Joseph Johnston, and Gerhardt Pohl are employees of Eli Lilly and Company.
Kathleen Foley, Sara Wang, and Stacey Long are employees of Thomson Medstat which received funding from Eli Lilly and Company to conduct the analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Foster, S.A., Foley, K.A., Meadows, E.S. et al. Characteristics of patients initiating teriparatide for the treatment of osteoporosis. Osteoporos Int 19, 373–377 (2008). https://doi.org/10.1007/s00198-007-0455-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-007-0455-4