Abstract
Summary
Weekly treatment of gonad-intact cynomolgus monkeys (for up to 6 months) with the RANKL inhibitor OPG-Fc reduced bone turnover markers and increased volumetric cortical and trabecular BMD and BMC at radial and tibial metaphyses. OPG-Fc was well tolerated in this study without evidence of change in measured toxicologic parameters vs. control.
Introduction
RANKL is the primary mediator of osteoclast formation, function, and survival. The catabolic effects of RANKL are inhibited by OPG, a soluble decoy receptor for RANKL. We investigated the safety and pharmacology of OPG-Fc in gonad-intact cynomolgus monkeys.
Methods
Males and females were treated weekly with vehicle (n = 5/sex) or OPG-Fc (15 mg/kg) by s.c. (n = 5/sex) or i.v. (n = 3/sex) injection for 6 months.
Results
Routine toxicologic investigations, hematologic parameters, body and organ weights, and ophthalmologic and electrocardiographic findings were not affected by OPG-Fc treatment. Because s.c. and i.v. dosing of OPG-Fc caused similar effects, these groups were combined for analyses. The following endpoints were significantly different in males and/or females treated with OPG-Fc relative to sex-matched vehicle controls after 6 months (p < 0.05). Biochemical markers of bone turnover (urine N-telopeptide and serum osteocalcin) were significantly decreased with OPG-Fc treatment. Cortical and trabecular volumetric BMD and BMC, cortical thickness, and cross-sectional moment of inertia were significantly increased by OPG-Fc treatment at the proximal tibia and distal radius metaphyses. Increases in cortical thickness were associated with significantly greater periosteal circumference.
Conclusions
OPG-Fc increased cortical and trabecular BMD and BMC in young gonad-intact cynomolgus monkeys.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
RANKL is a physiologic and pathologic mediator of osteoclast formation, function, and survival. RANKL has direct catabolic effects on cancellous and cortical bone [1]. The catabolic effects of RANKL are prevented by OPG, a soluble decoy receptor for RANKL that inhibits osteoclast formation, function, and survival [2–4]. Native OPG has a very brief circulating half-life [2], which is related in part to its heparin-binding domain. Deletion of this domain, and fusion of the truncated molecule to the Fc portion of human IgG1, confers a long circulating half-life that allows OPG-Fc to cause sustained but reversible suppression of bone turnover in rats [5]. Recombinant OPG-Fc has been shown to protect bone in rodent models of osteoporosis [6], rheumatoid arthritis [7], and humoral hypercalcemia of malignancy [8]. OPG-Fc also caused rapid and sustained suppression of bone turnover markers in patients with multiple myeloma or bone metastases from breast cancer [9]. However, the effects of OPG-Fc on bone mass, bone density, and cortical geometry have not been previously described in primates.
The cynomolgus monkey represents a good preclinical model for human bone biology because of similarities in skeletal anatomy and the presence of haversian osteonal remodeling in cortical bone [10]. OPG has been shown to improve cortical bone density, thickness, and strength in rodents [11–14]. Unlike primates, rat cortical bone lacks haversian systems and does not typically undergo significant cortical bone remodeling. It is therefore important to establish whether RANKL inhibition can increase cortical bone density and geometry in primates. Volumetric BMD (vBMD), which reflects both bone matrix mineralization and porosity, has been positively correlated with the load capacity of human cortical bone cylinders [15]. Cortical vBMD was previously shown to increase in cynomolgus monkeys treated with Fc-OPG, a first-generation OPG therapeutic construct with a shorter half-life compared with OPG-Fc [16]. Cortical geometry is another important attribute of the overall strength of long bones. In general, wider bones are stronger than narrow bones by virtue of an increased cross-sectional moment of inertia (CSMI). This parameter describes the distribution of bone mass around a central axis and is calculated from periosteal and endocortical dimensions. CSMI is well correlated with bone strength [17]. A small increase in periosteal dimensions can translate into an exponential (fourth-power) increase in CSMI. The effects of RANKL inhibition on CSMI have not been previously evaluated in large species with intracortical remodeling.
The purpose of this study was to examine the safety and efficacy of OPG-Fc treatment for 6 months in young, intact cynomolgus monkeys. In the absence of any meaningful toxicologic findings, we describe the effects of OPG-Fc treatment on biochemical markers of bone turnover, cortical geometry, and cortical and trabecular BMD and BMC.
Materials and methods
Animals
Juvenile male (n = 13) and female (n = 13) cynomolgus monkeys (2–3 years old) were obtained from Covance Research Products Inc. (Denver, PA, USA). Animals received commercial primate food (Laboratory Fiber-Plus Monkey Diet Jumbo 5050 with 0.91% calcium and 6.6 IU/g vitamin D3, PMI Feeds, Inc., St. Louis, MO, USA) twice daily, and tap water ad libitum. Animals were given an acclimation period of approximately 9 weeks, during which their health status and normal skeleton were verified. Three weeks before initiation of treatment, males and females were assigned to treatment groups that ensured similar mean body weights between sex-matched groups. This study was conducted in compliance with the guiding principles of the Institutional Animal Care and Use Committee (IACUC) of Charles River Laboratories Preclinical Services Montreal (Senneville, Quebec, Canada) and conducted under Good Laboratory Practice guidelines.
Study design
Animals were randomized to receive once-weekly doses of PBS (n = 5/sex) or OPG-Fc 15 mg/kg s.c. (n = 5/sex) or OPG-Fc 15 mg/kg i.v. (n = 3/sex). This genetically engineered OPG-Fc fusion protein was recombinantly produced in Chinese hamster ovary (CHO) cells, and consisted of the Fc fragment of human immunoglobulin G1 attached to the N-terminus of OPG as previously described [5]. The 15-mg/kg dose used in this toxicology study was 5-fold higher than the maximum dose used in previous human clinical trials [18]. Subcutaneous injections were given in the scapular region, and bolus i.v. injections were administered via the saphenous vein. After 6 months of treatment, animals were euthanized and tissues were collected for toxicology assessment and bone pharmacology endpoints.
Clinical observations and toxicologic and clinical chemistry analyses
All animals were examined twice daily for mortality and signs of morbidity. Body weight was recorded weekly, and food consumption was assessed twice daily by visual inspection approximately 1 hour after each feeding period. Electrocardiography (ECG) was performed before treatment and after 1, 3, and 6 months of treatment. Tracings were evaluated by a board-certified cardiologist. Ophthalmology testing, including funduscopic (indirect ophthalmoscopy) and biomicroscopic (slit-lamp) examinations, was carried out by an ophthalmologist before treatment and after 3 and 6 months of treatment. Standard hematology and clinical biochemistry panels were conducted on blood samples taken before treatment and after 3 and 6 months.
Biochemical markers of bone turnover
Biochemical markers of bone turnover were measured in the urine and serum at baseline, after 3 and 6 months of treatment. Urine parameters examined were N-telopeptide (NTx) (Osteomark ELISA kit, Ostex International, Inc., Seattle, WA, USA), and creatinine (via a kinetic Jaffe method on a Cobas Fara autoanalizer, Roche Diagnostic Systems, Somerville, NJ, USA).
Serum parameters examined were osteocalcin (DSL-6900 RIA kit, Diagnostic Systems Laboratories, Inc., Webster, TX, USA), and parathyroid hormone (PTH) 1-84 (Coat-A-Count Intact PTH IRMA kit, Diagnostic Products Corp., Los Angeles, CA, USA). Blood ionized calcium was measured using a NOVA 8 analyzer (Nova Biomedical, Waltham, MA, USA).
Peripheral quantitative computed tomography (pQCT)
Volumetric BMD, BMC, and geometric parameters of the right distal radius and right proximal tibia were measured by pQCT (Stratec XCT Research SA, software version 5.40, Norland Medical Systems, Inc., Fort Atkinson, WI, USA). Image resolution was 0.2 × 0.2 mm for radial scans and 0.35 × 0.35 mm for tibial scans. Measurements were made at baseline and after 3 and 6 months of treatment. Briefly, metaphyseal scans (3 slices, 0.5 mm apart) were taken beginning at approximately 9% of the bone length of the tibia (using the end of the tibia as the anatomical landmark) and 5% of the length of the radius (using the end of the ulna as the anatomical landmark). These respective regions were chosen to avoid primary spongiosa, a compartment that was previously shown to be preserved by OPG-Fc treatment in young, growing rodents [5]. Metaphyseal scans were analyzed for trabecular bone using the central 45% of the total bone slice (Peelmode 1) and for cortical bone using a 0.930-l/cm threshold (Cortmode 2). Diaphyseal scans were taken at approximately 22% of the bone length (from the proximal end for the tibia and distal end for the radius) and analyzed for cortical bone using a 0.930-l/cm threshold (Cortmode 2). Metaphyseal scans were evaluated for total slice, trabecular, and cortical vBMC and vBMD, as well as cortical geometry, whereas diaphyseal scans were evaluated for cortical parameters only.
Terminal procedures, gross pathology, and histopathology
Organs were weighed prior to termination. Histopathologic assessment was performed on the following tissues and organs: adrenals, aorta (thoracic), bone and marrow (sternum), brain, bronchi, cecum, colon, duodenum, epididymides, esophagus, eyes, gallbladder, gross lesions, heart (including section of aorta), ileum, injection site(s), jejunum, kidneys, liver, lungs, lymph nodes (mandibular and mesenteric), mammary gland (ventral thoracic), optic nerves, ovaries, pancreas, pituitary gland, prostate gland, salivary gland (submandibular), sciatic nerve, seminal vesicles, skeletal muscle, skin (ventral thoracic), spinal cord (cervical and thoracic), spleen, stomach (glandular and nonglandular mucosa), testes, thymus, thyroid lobes (and parathyroids), tongue, tonsil, trachea, ureters, urethra, urinary bladder, uterus (and cervix), and vagina. These tissues were fixed and preserved in 10% neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
Statistical methods
Group means and standard deviations were calculated from raw data for biochemical markers of bone turnover, and percent change from baseline for pQCT data. No significant differences were found using unpaired t-tests between the s.c. and i.v. treated animals in biomarkers or densitometry parameters; therefore, the s.c. and i.v. groups were combined for analysis. Unpaired t-tests were performed to compare OPG-Fc-treated animals with their sex-matched vehicle-treated controls at 3 and 6 months. Data included in the text are expressed as mean ± SE.
Results
Clinical observations and clinical pathology
No animals died during the treatment period, and no OPG-Fc-related clinical signs were noted. No significant treatment related differences were found in body weight or organ weights, and no changes were noted by ophthalmologic or ECG evaluation. Average weight gain during the 6 month observation period was similar between groups treated with OPG-Fc or vehicle (16.9 ± 4.6% and 15.2 ± 2.5% gain in females treated with OPG-Fc or vehicle, respectively, p = 0.79; 19.5 ± 4.2% and 17.9 ± 4.4% gain in males treated with OPG-Fc or vehicle, respectively, p = 0.80). Longitudinal growth of the tibia continued during the 6 month evaluation period, and was similar between groups treated with OPG-Fc or vehicle (9.0 ± 1.2% and 9.4 ± 1.6% increase in females treated with OPG-Fc or vehicle, respectively, p = 0.85; 7.9 ± 1.6% versus 7.7 ± 1.1% increase in males treated with OPG-Fc or vehicle, respectively, p = 0.93).
Hematology and clinical biochemistry
Results of laboratory tests and hematologic assessments showed no clinically relevant, toxicologically meaningful changes or differences between groups during the treatment period. Although slight reductions in mean red blood cell count and hemoglobin concentration were noted at months 3 and 6 in males who received OPG-Fc, no significant differences were found relative to pretreatment baseline values (data not shown). Significant decreases in blood ionized calcium were observed with OPG-Fc treatment at months 3 and 6 in females and at month 6 in males (Table 1).
Gross pathology and histopathologic examination
No toxicologically meaningful, treatment-related macroscopic changes were found at necropsy, and organ weights were normal across all groups. Histologic assessment showed that OPG-Fc treatment led to the expected accrual of primary spongiosa at the growth plates of the sternum, consistent with its mechanism of action in growing animals [16].
OPG-Fc decreased bone turnover and increased serum PTH
OPG-Fc treatment was associated with significant reductions in biochemical markers of bone turnover at months 3 and 6, as summarized in Table 1. After 3 months of OPG-Fc treatment, the resorption marker urine NTx was significantly reduced in both males and females (>90% below vehicle controls, p < 0.05). This level of suppression persisted through 6 months of treatment. Endogenous serum PTH levels in OPG-treated males and females were significantly increased at months 3 and 6, presumably in response to the treatment-related reduction in calcium release from bone. The bone formation marker osteocalcin was significantly reduced at months 3 (>55%) and 6 (>73%) in males and females treated with OPG-Fc (p < 0.05 vs. vehicle controls).
OPG-Fc increased trabecular vBMD and vBMC in long bone metaphyses
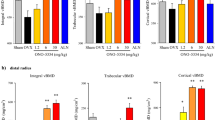
OPG-Fc treatment was associated with significant increases in trabecular vBMD and vBMC at the distal radius in both males and females (Fig. 1a and c). These increases were noted after 3 and 6 months of treatment, and were similar in both sexes. OPG-Fc treatment also increased vBMD and vBMC in the proximal tibia of males and females, although the increase in vBMD in females did not reach statistical significance at the proximal tibia (Fig. 1b and d).
Trabecular volumetric BMD (vBMD) and BMC (vBMC) were analyzed in proximal tibia and distal radius metaphyseal scans using the central 45% of the total bone slice. Trabecular vBMD was significantly increased at both sites in male cynomologus monkeys treated with OPG-Fc for 6 months, but only at the radius in female monkeys. Trabecular vBMC was significantly increased at both sites in male and female cynomologus monkeys. Data points represent group mean ± SE, n = 5 for vehicle groups, n = 8 for OPG-Fc groups. *p < 0.05 vs. vehicle for females. † p < 0.05 vs. vehicle for males
OPG-Fc increased cortical vBMD and vBMC while improving cortical geometry in long bone metaphyses
OPG-Fc treatment was associated with significantly greater vBMD and vBMC in the cortical compartment of the distal radius and proximal tibia after 3 and 6 months (p < 0.05 vs. sex-matched vehicle controls, Fig. 2a–d). OPG-Fc treatment also led to significantly greater cortical area and thickness at these two sites (Fig. 3a–d). These increases were statistically significant at months 3 and 6 for the distal radius, and at month 6 for the proximal tibia. Increased cortical thickness and area at both skeletal sites was associated with geometric changes at the endocortical and periosteal surfaces. The endocortical circumference of the distal radius was significantly reduced by OPG-Fc treatment in males and females after 6 months of treatment (Fig. 4a). OPG-Fc did not reduce endocortical circumference in the proximal tibia of males or females (Fig. 4b). Periosteal circumference of the distal radius was significantly greater in males and females treated with OPG-Fc (Fig. 4c). At the proximal tibia, males treated with OPG-Fc had significantly greater periosteal circumference while females showed a trend toward greater circumference that did not reach statistical significance (Fig. 4d). These geometric changes led to a significant increase in the CSMI for both the distal radius and proximal tibia in males and females after 3 and 6 months of OPG-Fc treatment (p < 0.05 vs. sex-matched vehicle controls, Fig. 5a and b). CSMI is a geometric endpoint that describes the distribution of bone around a central axis, and a greater CSMI is usually associated with greater resistance to bending forces. CSMI does not account for the density of cortical bone, which may also be an important independent attribute of cortical strength. To capture the potential contribution of cortical density to bone strength, CSMI was multiplied by its respective vBMD value. The product of these two variables has been described previously as an estimate of bone strength, called the bone strength index (BSI) [19]. OPG-Fc treatment was associated with significant increases in BSI of the distal radius and proximal tibia in males and females after 3 and 6 months of treatment (Fig. 5c and d).
Cortical volumetric BMD (vBMD) and BMC (vBMC) were analyzed in proximal tibia and distal radius metaphyseal scans using a 0.930−l/cm threshold. In both male and female monkeys, cortical vBMD and vBMC were significantly increased after 3 and 6 months of OPG-Fc treatment (vs. vehicle controls). Data points represent group mean ± SE, n = 5 for vehicle groups, n = 8 for OPG-Fc groups. *p < 0.05 vs. vehicle for females. † p < 0.05 vs. vehicle for males
Cortical thickness and area were analyzed in proximal tibia and distal radius metaphyseal scans using a 0.930−l/cm cortical threshold. In both male and female monkeys, cortical thickness and area were significantly increased at both sites after 6 months of OPG-Fc treatment (vs. vehicle controls). Data points represent group mean ± SE, n = 5 for vehicle groups, n = 8 for OPG-Fc groups. *p < 0.05 vs. vehicle for females; † p < 0.05 vs. vehicle for males
Endocortical and periosteal circumference were analyzed in proximal tibia and distal radius metaphyseal scans using a 0.930-l/cm cortical threshold. Periosteal circumference was significantly increased in OPG-Fc treated male and female monkeys at the radius, and in males only at the proximal tibia. Radial endocortical circumference was significantly reduced in OPG-Fc treated monkeys. Data points represent group mean ± SE, n = 5 for vehicle groups, n = 8 for OPG-Fc groups. *p < 0.05 vs. vehicle for females. † p < 0.05 vs. vehicle for males
Cross-sectional moment of inertia (CSMI) and bone strength index (BSI) at metaphyses were analyzed in proximal tibia and distal radius metaphyseal scans using a 0.930-l/cm cortical threshold. CSMI was calculated based on a circular geometry assumption from periosteal and endocortical circumferences. BSI was calculated as the product of CSMI and cortical volumetric BMD (vBMD). In both male and female monkeys, CSMI and BSI were significantly increased after 3 and 6 months of OPG-Fc treatment (vs. vehicle controls). Data points represent group mean ± SE, n = 5 for vehicle groups, n = 8 for OPG-Fc groups. *p < 0.05 vs. vehicle for females. † p < 0.05 vs. vehicle for males
OPG-Fc increased cortical vBMD and vBMC in long bone diaphyses
The diaphyses of the radius and tibia were also assessed by pQCT to examine treatment-related changes at purely cortical sites. OPG-Fc treatment was associated with significantly greater vBMD in the diaphyses of both bones, in males and in females, after 3 and 6 months (p < 0.05 vs. sex-matched vehicle controls, Fig. 6a and b). Table 2 demonstrates the changes in geometric and densitometric endpoints at the tibia diaphysis during the 6-month treatment period. OPG-Fc treatment caused modest increases in tibia diaphyseal vBMC, achieving statistical significance in males (Table 2). Cortical thickness and area were modestly and non-significantly increased with OPG-Fc treatment in males and females compared to vehicle controls (Table 2). No treatment related differences were found in CSMI or periosteal circumference at the diaphysis of the radius or tibia, although both parameters increased with age (Table 2). BSI at the tibial diaphysis was significantly increased in males (p = 0.028).
Cortical volumetric BMD (vBMD) was analyzed in tibia and radius diaphyseal scans using a cortical 0.930-l/cm threshold. In both male and female monkeys, cortical vBMD was significantly increased after 3 and 6 months of OPG-Fc treatment (vs. vehicle controls). Data points represent group mean ± SE, n = 5 for vehicle groups, n = 8 for OPG-Fc groups. *p < 0.05 vs. vehicle for females. † p < 0.05 vs. vehicle for males
Discussion
This is the first report that describes the effects of 6 months of RANKL inhibition on cortical and cancellous BMD and BMC and cortical geometry in nonhuman primates. This study was intended to assess the safety and tolerability of OPG-Fc, a RANKL inhibitor with a longer half-life relative to native full-length OPG [2]. Consistent with a shorter-term primate study using a different RANKL inhibitor (Fc-OPG) [16], there were no significant toxicologic effects of OPG-Fc in this study.
Treatment with OPG-Fc was associated with significant, sustained, and persistent reductions in biochemical markers of bone turnover. Reductions in bone resorption markers with OPG-Fc treatment were consistent with the ability of OPG to inhibit osteoclast formation, function, and survival [2–4]. The decrease in systemic bone formation markers was an expected pharmacologic response to the suppression of bone resorption, because of normal coupling of formation and resorption on remodeling surfaces. This coupling has been shown to be preserved on remodeling surfaces during RANKL inhibition [20]. Despite the systemic reduction in bone formation markers, OPG-Fc treatment was not associated with differences in longitudinal bone growth. OPG-Fc treatment was associated with significantly greater increments in cortical and trabecular vBMC compared with vehicle-treated animals. Furthermore, pQCT analysis indicated an absolute increase in periosteal circumference, as well as a reduction in endocortical circumference at the distal radial metaphysis.
In the absence of bone histomorphometry from these animals, it is possible that some of these pQCT-derived changes in cortical geometry were related at least in part to a treatment-related increase in bone matrix mineralization. Previous studies showed that matrix mineralization increased with OPG treatment [21], and increased mineralization has the potential to confound the interpretation of pQCT-derived geometric endpoints due to partial volume effects. Matrix mineralization appeared to increase with OPG-Fc treatment in this study as well, as suggested by significant increases in vBMD in the diaphyses of long bones. However, the magnitude of the changes in geometric endpoints observed in this primate study suggests that partial volume effects did not account for all of these changes. It is reasonable to assume that the treatment-related accrual of bone mass in the current study was related to a combination of increased matrix density and increased matrix volume.
The periosteal and endocortical changes associated with OPG-Fc treatment resulted in significantly greater cortical area, thickness, and CSMI at the distal radius and proximal tibia. The observed reduction in endocortical circumference with OPG-Fc would be consistent with the suppression of endocortical bone resorption. The observed treatment-related increases in periosteal circumference of long bone metaphyses were probably related, at least in part, to the young age and open growth plates in these young primates. Metaphyseal geometry is more influenced by modeling-dependent bone growth compared to diaphyseal sites, and the tibial diaphysis showed only modest geometry improvements with OPG-Fc treatment. The lack of significant treatment-related changes in diaphyseal geometry might have been influenced by the relatively short (6 month) duration of this study. Increases in cortical thickness and area have been observed at the femur midshaft of older (4–5 year old) intact cynomolgus monkeys treated for 12 months with a different RANKL inhibitor (denosumab, a fully human RANKL antibody) [22]. Furthermore, a study in postmenopausal women recently demonstrated that 24 months of therapy with denosumab led to a significant apparent increase in the cross-sectional area of the proximal femur diaphysis [23]. These observations suggest that the ability of RANKL inhibitors to improve cortical geometry is not confined to growing and/or gonad-intact animals. However, it is also clear that the magnitude and rate of treatment-related densitometric and geometric changes observed in young growing primates, as described in this study, should not be anticipated in postmenopausal subjects.
A potential limitation of this study was the use of a relatively high dose of OPG-Fc (15 mg/kg), which is approximately fivefold greater than the highest dose tested in clinical trials [18]. Human OPG-Fc is a foreign protein in cynomolgus monkeys, so a high dose was chosen to overwhelm potential immune responses and thereby maintain drug exposure throughout the 6-month experiment. This goal was achieved, as demonstrated by the sustained and marked (88–95%) suppression of urine NTx in OPG-treated animals. This level of suppression was similar to the maximal 90% suppression observed in a previous cynomolgus monkey study after a single injection of 1 mg/kg human OPG-Fc (Amgen data on file). It is, therefore, unlikely that doses greater than 1 mg/kg would lead to exaggerated pharmacologic responses in cynomolgus monkeys.
Another limitation is that this study did not evaluate bone strength by destructive biomechanical testing. In the absence of such data, we attempted to estimate long bone strength by multiplying CSMI by cortical vBMD, as previously described [19]. The resulting bone strength index (BSI) was significantly greater with OPG-Fc treatment in both males and females at the radial and tibial metaphyses, as early as 3 months after the initiation of therapy. At the tibial diaphysis, OPG-Fc treatment was associated with significantly greater BSI in males compared to vehicle treated controls. Although this surrogate of bone strength has not been validated in primate species, other studies in rats have shown significant improvements in actual bone strength with OPG treatment, including increases in the strength of the femoral neck [12] and diaphysis [12, 13]. Interpretation of data from these rat studies, and from the current study, is limited by the substantial contribution of growth-related bone modeling to changes in bone density and geometry. From a clinical perspective, it is imperative to confirm the ability of RANKL inhibitors to improve long bone strength in aged skeletally mature animals with intracortical remodeling. Such studies are currently underway in aged ovariectomized nonhuman primates treated with denosumab. The effect of denosumab on fracture incidence is also being evaluated in postmenopausal women with osteopenia and osteoporosis.
In summary, 6 months of RANKL inhibition with OPG-Fc resulted in significant suppression of bone turnover markers and significant increases in cortical and trabecular vBMD and vBMC in young gonad-intact cynomolgus monkeys. OPG-Fc treatment was also associated with increases in cortical thickness, area, and CSMI. The ability of OPG-Fc to increase the density and apparent volume of cortical and cancellous bone in nonhuman primates supports the further investigation of RANKL inhibition as a therapeutic strategy for patients with low bone mass or other risk factors for fracture.
References
Mizuno A, Kanno T, Hoshi M et al (2002) Transgenic mice overexpressing soluble osteoclast differentiation factor (sODF) exhibit severe osteoporosis. J Bone Miner Metab 20:337–344
Simonet WS, Lacey DL, Dunstan C et al (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Burgess T, Qian YX, Kaufman S et al (1999) The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol 145:527–538
Lacey DL, Tan HL, Lu J et al (2000) Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol 157:435–448
Capparelli C, Morony S, Warmington K et al (2003) Sustained antiresorptive effects after a single treatment with human recombinant osteoprotegerin (OPG): a pharmacodynamic and pharmacokinetic analysis in rats. J Bone Miner Res 18:852–858
Bolon B, Carter C, Daris M et al (2000) Adenoviral delivery of recombinant osteoprotegerin (OPG) ameliorates bone resorption in a mouse ovariectomy model of osteoporosis. Mol Ther 3:179–205
Stolina M, Adamu S, Ominsky MS et al (2005) RANKL is a marker and mediator of local and systemic bone loss in two rat models of inflammatory arthritis. J Bone Miner Res 20:1756–1765
Morony S, Warmington K, Adamu S et al (2005) The RANKL inhibitor osteoprotegerin (OPG) causes greater suppression of bone resorption and hypercalcemia compared to bisphosphonates in two models of humoral hypercalcemia of malignancy. Endocrinology 146:3235–3243
Body JJ, Greipp P, Coleman RE et al (2003) A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer Suppl 3:892
Jerome CP, Peterson PE (2001) Nonhuman primate models in skeletal research. Bone 29:1–6
Bateman TA, Dunstan CR, Ferguson VL et al (2000) Osteoprotegerin mitigates tail suspension-induced osteopenia. Bone 26:443–449
Ross AB, Bateman TA, Kostenuik PJ et al (2001) The effects of osteoprotegerin on the mechanical properties of rat bone. J Mater Sci Mater Med 12:583–588
Kostenuik PJ, Capparelli C, Morony S et al (2001) OPG and PTH-(1–34) have additive effects on bone density and mechanical strength in osteopenic ovariectomized rats. Endocrinology 142:4295–4305
Ichinose Y, Tanaka H, Inoue M et al (2004) Osteoclastogenesis inhibitor factor/osteoprotegerin reduced bone loss induced by mechanical unloading. Calcif Tissue Int 75:338–343
Wachter NJ, Krischak GD, Mentzel M et al (2002) Correlation of bone mineral density with strength and microstructural parameters of cortical bone in vitro. Bone 31:90–95
Smith BB, Cosenza ME, Mancini A et al (2003) A toxicity profile of osteoprotegerin in the cynomolgus monkey. Int J Toxicol 22:403–412
Myers ER, Hecker AT, Rooks DS et al (1993) Geometric variables from DXA of the radius predict forearm fracture load in vitro. Calcif Tissue Int 52:199–204
Bekker PJ, Holloway D, Nakanishi A et al (2001) The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res 16:348–360
Ferretti JL, Capozza RF, Zanchetta JR (1996) Mechanical validation of a tomographic (pQCT) index for noninvasive estimation of rat femur bending strength. Bone 18:97–102
Nakamura M, Udagawa N, Matsuura S et al (2003) Osteoprotegerin regulates bone formation through a coupling mechanism with bone resorption. Endocrinol 144:5441–5449
Valenta A, Roschger P, Fratzl-Zelman N et al (2005) Combined treatment with PTH (1–34) and OPG increases bone volume and uniformity of mineralization in aged ovariectomized rats. Bone 37:87–95
Atkinson J, Cranmer P, Saunders T et al (2005) AMG 162, a fully human RANKL antibody, increases bone mass and bone strength in cynomolgus monkeys. J Bone Min Res 20(Suppl 1):S294, (abstract)
Beck TJ, Miller PD, Lewiecki EM et al (2006) Denosumab improves structural geometry of the proximal femur in postmenopausal women with low bone mass. J Bone Min Res 21(Suppl 1):S71, (abstract)
Funding
Research support provided by Amgen Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ominsky, M.S., Kostenuik, P.J., Cranmer, P. et al. The RANKL inhibitor OPG-Fc increases cortical and trabecular bone mass in young gonad-intact cynomolgus monkeys. Osteoporos Int 18, 1073–1082 (2007). https://doi.org/10.1007/s00198-007-0363-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-007-0363-7