Abstract
Introduction and hypothesis
A large number studies have examined the association between estrogen receptor alpha (ESR-α) gene polymorphisms and bone mineral density (BMD) in the Chinese population. We conducted a meta-analysis to assess their pooled effects.
Methods
We searched for all published articles indexed in MEDLINE, the Chinese Biomedical Database, and the Chinese Journal Full-text Database from January 1994 to April 2006. Any cross-sectional study that tested the association between ESR-α PvuII or XbaI genotypes and BMD at the femoral neck or spine in Chinese women was included in the review. Data were extracted independently by two reviewers using a standardized data extraction form. Sixteen eligible studies involving 4,297 Chinese women were identified.
Results
The overall frequencies of X and P alleles were 28% and 40%, respectively. The PvuII polymorphism was statistically significantly associated with BMD at the femoral neck (P = 0.038 for PP = Pp = pp) but not at the lumbar spine in all women. The BMD difference for the contrasts of PP versus Pp/pp genotypes was −0.0105 (95%CI, −0.0202 ∼ −0.0008) g/cm2 (P = 0.036). The XbaI polymorphism was not associated with BMD at the femoral neck or lumbar spine.
Conclusion
The PvuII polymorphism had a very weak association with femoral neck BMD whereas XbaI polymorphism was unlikely to be a predictor of femoral neck or spine BMD in Chinese women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is an important public health problem affecting both the Western and Asian populations. It affects one in three postmenopausal women and the majority of the elderly. Bone mineral density (BMD) is the major determinant of fragility fracture [1]. Although many environmental factors, such as dietary intakes, physical activities, education, etc., play an important role in BMD, it is strongly inherited. From studies of monozygotic and dizygotic twins, inheritance was estimated to account for 60–80% of BMD in both men [2] and women [3, 4]. In this regard, a large number of polymorphisms in multiple candidate genes have been investigated in Caucasian [5, 6] as well as in Chinese women [7–53]. Of them, estrogen receptor alpha (ESR-α) genes—in particular, those defined by the restriction enzymes XbaI and PvuII [5, 6]—have been among of the most intensively studied gene polymorphisms in the genetic regulation of BMD [54]. Due to limited sample size and different inferences, inconsistent results were generated from the individual studies. Two meta-analyses examined the pooled effects of ESR-α gene polymorphisms on osteoporosis outcomes and found the ESR-α gene was associated with fracture risks [6] or BMD [5]. However, since the majority of relevant studies in the Chinese population were published in local Chinese journals [7–10, 12, 14–21, 23, 25, 27–41, 43–45, 50–53], most international readers could not reach and/or read these Chinese articles. The meta-analysis by Ioannidis et al. [5] has thus included only those studies of Chinese populations that published in international journals [11, 13, 24].
Due to potential gene–gene and gene–environment interactions [54] and differences in potential confounders between Chinese and Caucasian populations, it is still unclear whether the effect of ESR-α gene polymorphisms on BMD differs between Chinese and Caucasians. This meta-analysis aims to pool molecular association studies addressing the relationship between ESR-α gene polymorphisms and BMD in Chinese women.
Materials and methods
Search strategy
We searched for all published articles indexed in MEDLINE, the Chinese Biomedical Database (CBMDisc), and the Chinese Journal Full-text Database (CJFD), and the time frame was limited from January 1994 to April 2006. Literature searches were performed by an expert using the keywords in title, abstract, or keywords. The keywords were as follows: (1) estrogen receptor or ESR-α gene or XbaI or PvuII; (2) gene or genotype(s) or allele(s) or polymorphism(s); (3) bone mineral density or BMD or bone density; (4) Chinese or China or Hong Kong or Taiwan or Taiwanese; (5) (1) and (2) and (3) and (4). We accepted studies written in English or Chinese of Chinese women aged 18 years or over. We also perused the bibliographies of retrieved articles. We used the most complete and first results with the most participants when there were duplicate publications from the same study group.
Inclusion and exclusion criteria
Observational studies (cross-sectional studies only) that tested the association between ESR-α gene polymorphisms and BMD at the lumbar spine or femoral neck or both and that fulfilled the following criteria were qualified for inclusion: (1) BMD was measured by dual-energy X-ray absorptiometry (DXA); (2) genotyping was performed with polymerase chain reaction (PCR), and the possible genotypes were PP, Pp, pp for PvuII and XX, Xx, xx for XbaI, where P and X indicated absence of the restriction site; (3) result description included number of subjects, and the mean and standard deviation (SD) of BMD for each genotype of ESR-α gene; and (4) participants were Chinese women without chronic diseases or conditions that may potentially affect BMD and without chronic use of any drugs affecting bone metabolism.
We assumed the publications were generated from the same studies when they were done by the same research group at the same setting using the same subject recruitment methods, and the study population had similar characteristics (e.g., age, gender). For these multiple publications of the same studies in different Chinese and/or English journals, we only included the first publication in the duplicates with the same study size, or the publication with the largest study size among those with varied study sizes. The same data were used only once in the same analysis.
Data extraction
For each eligible study, we extracted information on authors, year of publication, age (range or mean and SD), menopause status, the instrument used for BMD measurement, the number of subjects with both BMD data and genotype, inclusion criteria, the mean (SD) of BMD in each genotype, and the frequency of the P and X alleles (if available). All data were extracted independently by two reviewers using a standard form, and minor discrepancies were resolved by authors’ discussion.
Statistical analysis
We pooled eligible studies according to site of BMD measurement and performed analyses at the lumbar spine and femoral neck separately. BMD at other skeletal sites was not analyzed due to the small number of subjects. The main analysis examined differences in BMD between different genotypes. Genotype contrasts were listed as follows: PP versus Pp, PP versus pp, and Pp versus pp for PvuII polymorphism; XX versus Xx, XX versus xx, and Xx versus xx for XbaI polymorphism. Contrasts of one genotype versus the combination of two others were also tested when the combination seemed appropriated based on the pairwise comparisons. We estimated the difference in BMD between the contrasted genotypes and the SD of the difference for each included study group. Between-study heterogeneity was assessed separately for the differences of means for included studies. Results without heterogeneity were pooled using the fixed-effects model; otherwise, the random-effects model was used. We checked the studies for Hardy-Weinberg equilibrium (HWE).
We used the SAS MIXED Procedure, version 9.1 (SAS Institute, Cary, NC, USA) to determine whether the ESR genotypes could significantly explain BMD, as described in previous studies [55, 56]. The dependent variable in the analysis was the mean BMD in each genotype group. The independent variable was genotype, and the modulator variables were scanner types (QDR-2000/4500, XR-36, and DPX-L) and age (in years). Only covariates that remained significant or were borderline significant (p < 0.10) were retained in the final model. Manual backward stepwise method was used in removing the potential covariates (F-to- remove criteria were 0.10). Study and study subgroup were defined as random-effects variables. The weighted least-squares method was used to determine the main difference in the mean level of BMD between genotypes, with weights proportional to the inverse of the variance (1/se 2) of the mean of each group in each study. Results with a p value less than 0.05 were considered statistically significant, with the exception of tests of heterogeneity, where the statistically significant level was 0.10.
We also did sensitivity analyses by limiting to (1) postmenopausal women, (2) postmenopausal women in HWE, and (3) premenopausal women. Publication bias was checked using inverted funnel plots. Weighted least squares meta-regression was conducted to identify determinants of the between-study/group heterogeneity. In the meta-regression, the outcome variable was the effect estimate (BMD difference of each genotype contrast). The explanatory variables were mean age, gender, menopause status, and DXA instruments. Categorical variables were transferred into dummy variables. Regression was weighted by the reciprocal of se 2 of the mean BMD differences.
Results
Eligible studies/groups
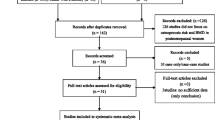
Forty-seven potentially eligible studies were identified [7–53]. Of those, 15 [23–37] were excluded because they seemed to be duplicates of other studies [7, 10, 12, 16–19, 21]. Six studies [38–43] reported BMD only as a Z-score and/or were duplicated with each other. Two studies [44, 45] reported BMD for distal radius only. Two studies did not report XbaI or PvuII polymorphism [48, 49]. Two studies only included diabetic patients [51] or pregnant women [52], two studies only reported BMD by haplotypes of XbaI and PvuII polymorphisms [50] or the pooled BMD of women and men [53], and two studies [46, 47] only reported correlation coefficients between ESR-α gene polymorphisms and BMD or p values for relevant genotype contrasts and seemed to be duplicate publications of another study [22]. Therefore, 16 eligible studies with 4,297 Chinese women from 24 study groups (Table 1) were considered in the analysis, as shows in Table 1 [7–22].
Characteristics of studies and subjects
All studies were of a cross-sectional design, and subjects with diseases or medications affecting BMD were excluded. Most study subjects were postmenopausal women. BMD from five studies [10, 11, 16, 17, 22] were adjusted for age, and years since menopause [10, 11, 16, 17], height and weight [11, 16, 17], years of menstruation [22], and job [16]. HWE of PvuII polymorphism in one study [11] and of XbaI polymorphism in one studies [8] was violated. The X allele frequency ranged from 14% to 50% (overall 28%), and the P allele frequency ranged between 31% and 48% (overall 40%) (Table 1).
PvuII polymorphism and BMD
In 3,979 and 3,205 subjects, the PvuII allele and BMD of the lumbar spine and femoral neck, respectively, were examined. The PvuII polymorphism was statistically significantly associated with BMD at the femoral neck but not at the lumbar spine. There was no significant between-study/group heterogeneity in any genotype contrasts at the femoral neck (p > 0.10 for all). Under the fixed-effects model, the mean BMD at the femoral neck was significantly lower in the PP versus Pp (p = 0.036) and the PP versus Pp/pp (p = 0.036) genotype groups in all women. BMD difference for the contrasts of PP versus Pp/pp was −0.0105 (95%CI, −0.0202 ∼ −0.0008) g/cm2. The difference was equivalent to about 1.5% of the mean (0.69 g/cm2) or 10% of the SD (0.1 g/cm2) of femoral neck BMD in Chinese women. No significant BMD difference at the femoral neck was observed between the PP/Pp and pp genotype groups in women (p > 0.2). We found significant between-study/group heterogeneity in PP versus Pp and in PP versus pp genotype groups in BMD differences at the spine (p < 0.1). No significant difference in spine BMD among PP, Pp, and pp genotypes, in PP versus Pp/pp, and PP/Pp versus pp genotypes was noted under both fixed- and random-effects models (p > 0.05) (Fig. 1, Table 2).
Weighted mean differences in bone mineral density (BMD) [in grams per centimeter squared (g/cm2) at the lumbar spine (a) and femoral neck (b) for the contrasts of PvuII genotypes in Chinese women. For details of procedures, see “Statistical analysis”
XbaI polymorphism and BMD
There were 3,446 and 2,668 subjects with data of XbaI genotype and BMD of the lumbar spine and femoral neck, respectively. Significant between-study/group heterogeneity for comparisons of XX versus Xx and XX versus xx at the lumbar spine and femoral neck (p < 0.10) was observed, even after excluding the studies in which the HWE was violated. Random-effects models were thus used to compare BMD differences. We did not observe statistically significant association between the XbaI polymorphism and BMD, regardless of the genetic contrasts or skeletal sites considered by fix-effects or random-effects models. XbaI polymorphism also had no apparent association with BMD when limited to HWE studies at the lumbar spine or femoral neck (Fig. 2, Table 3).
Weighted mean differences in bone mineral density (BMD) [in grams per meter squared (g/cm2)] at the lumbar spine (a) and femoral neck (b) for the contrasts of XbaI genotypes in Chinese women. For details of procedures, see “Statistical analysis”. * Each one subject of XX genotype in the An 2001-1 [7] and An 2001-2 [7] study groups
Sensitivity analyses and publication bias diagnostics
Sensitivity was also examined by subgroup analyses. BMD differences of genotype contrasts and between-study/group heterogeneity were largely similar when analyses were limited to postmenopausal women, postmenopausal women in HWE, and premenopausal women (Tables 2 and 3). The mean BMD at the femoral neck was statistically significantly lower in the carriers of the PP genotype compared with those of combined Pp/pp genotypes in the above subgroups (p < 0.05 for all) except for premenopausal women. The pooled differences in BMD at the femoral neck between PP and Pp/pp were −0.0114 (95%CI: −0.0228 ∼ −0.0000, p = 0.049) and −0.0113 (95%CI: −0.0224 ∼ −0.0002, p = 0.047) g/cm2 in all postmenopausal women and those limited to HWE, respectively. No statistically significant difference in BMD was observed among XbaI polymorphism contrasts at the lumbar spine or femoral neck and among PvuII polymorphism contrasts at the lumbar spine (p > 0.05). Subgroup analyses could not identify the causes of heterogeneity.
Among all subgroups except premenopausal women, comparisons of lumbar spine BMD between PP and pp and between XX and xx showed statistically significant between-study/group heterogeneity (p < 0.1). Weighted least squares meta-regression showed that different DXA instruments was a major determinant, which could explain about 40% of variation in BMD differences between PP and pp and XX and xx at the lumbar spine. Age and menopause status were also statistically significantly associated with the BMD differences but played a less important role than DXA instruments (data not shown). Publication bias was examined by analyzing funnel plots for all genotype contrasts. These plots were symmetrical, providing evidence against publication bias. An example of a funnel plot for femoral neck BMD is shown in Fig. 3, demonstrating symmetry for PP versus Pp/pp genotype comparison.
Discussion
This meta-analysis pooled the association between the ESR-α polymorphisms and BMD in 4,297 Chinese women with measurements of the lumbar spine and/or femoral neck BMD and PvuII and/or XbaI polymorphisms. The data suggested that PvuII polymorphism had a very weak but marginally statistically significant association with BMD at the femoral neck but not at the lumbar spine. PP homozygotes had lower BMD than did Pp/pp genotype groups by about 1.5% of the mean or 10% of SDs. This finding was consistently observed when analyses were limited to postmenopausal women and postmenopausal women in HWE. Our finding suggested that the PvuII polymorphism might be one of the candidate genetic markers responsible for femoral neck BMD in Chinese postmenopausal women. However, XbaI polymorphism was unlikely to be associated with BMD of the lumbar spine or femoral neck. This result was at variance with a previous meta-analysis based on combined data of Caucasians and Asians by Ioannidis et al. [5] in which they found no significant relationship between PvuII polymorphism and BMD, but they observed a significantly higher BMD and lower risk of fracture in subjects with XX homozygotes in comparison with carriers of the x allele. However, Ioannidis et al. [6] did not find significant association between ESR-α gene polymorphisms and BMD in another meta-analysis of individual-level data involving standardized genotyping of 18,917 subjects in eight European centers. The reason for this discrepancy is unclear. Chinese populations had a quite different lifestyle and also a varied distribution of vitamin D receptor (VDR) gene polymorphisms from Caucasians. Some studies found significant interactions of ESR–VDR, ESR–age, and ESR–calcium to BMD [57–59]. Therefore, the genetic effects of the ESR may be modulated by age, lifestyle, and other genes, although the exact mechanism underlying the associations we described here remained to be elucidated. Dvornyk et al. [46] also reported that candidate genes of bone mass may have different effects on bone mass between Caucasians and Chinese. Furthermore, the discrepancy may also be due to the fact that previous meta-analyses pooled despite the presence of heterogeneity.
In this study, we observed significant between-study/group heterogeneity in the association between the XbaI polymorphism and BMD at the lumbar spine and femoral neck. Large heterogeneity might cause pooled results to differ from the results of some individual studies/groups. We conducted subgroup and meta-regression analyses to identify heterogeneity. Subgroup analysis showed a similar trend in the association between the XbaI or PvuII polymorphisms and BMD in all women, postmenopausal women, and those in HWE. Meta-regression analysis found that different DXA instruments, age, and menopause status could accounted for about 50% of variation in BMD differences among individual studies for genotype contrasts. Therefore, different characteristics of studied subjects and different DXA instruments might be responsible for the discrepancy in overall estimates among meta-analyses.
The major determinants of BMD are body weight, height or body mass index, age, and menopause status. However, most studies did not adjust for such potential confounders, nor did they provide data by genotypes. Only five original studies adjusted for age, body height, body weight, years since menopause, or other factors in the calculation of mean BMDs and SDs [10, 11, 16, 17, 22]. Potential confounding bias in the original studies might also contribute to the between-study/group heterogeneity.
The overall frequency of the X allele (28%) and the P allele (40%) in the Chinese population included in this study was similar to those of Asians (23% and 41%) involved in the meta-analysis by Ioannidis et al. [5]. In this meta-analysis, all original studies were cross-sectional studies. The overall frequency of the X and P alleles of this study would be closer to the population frequency than that from case-control studies.
Previous studies suggest that the ESR-α gene might be important in the accretion of BMD during young adulthood [60], but the effect was lost after menopause [61]. However, this meta-analysis found a similar trend in pre- and postmenopausal women. The significance of the association between ESR-α gene polymorphisms and femoral neck BMD was more pronounced in postmenopausal women than in premenopausal women, possibly due to a much smaller sample size in the latter. Previous meta-analyses also observed a similar relationship in pre- and postmenopausal women [5, 6].
Generally consistent with the original studies [7, 8, 11–13, 15–22, 29, 30] and previous meta-analyses [5, 6], our findings showed only 1.5% or less of mean (or 10% of SD) BMD differences among the genotype contrasts of ESR-α gene polymorphisms. If each SD decrease in BMD causes a 50% increase in the risk of fractures, the ESR-α gene effect would translate into a 5% increase in the risk of fractures in those with the PP genotype compared with the Pp/pp genotypes. In this regards, ESR-α gene polymorphisms might have little impact on osteoporotic fractures in Chinese women.
Subjects in this study were recruited from communities, social centers, or clinics. They were in apparent good health and free of fractures, other diseases, or medications affecting bone mass. The influences of these factors on BMD difference for genotype contrasts would thus be excluded. One limitation was that three studies [11, 13, 24], including 882 Chinese women for the PvuII analysis and 704 Chinese women for the XbaI analysis, had been included in the previous relevant meta-analysis by Ioannidis et al. [5]. Since the inclusion criteria of this study included all Chinese population studied published both in English and Chinese, the above three studies were also included in this meta-analysis. About 20% of the original published data in our study overlapped with those in the previous meta-analysis [5].
Furthermore, the cut-off of p < 0.05 for statistical significance was meant to refer to when an experimenter conducted a specific planned study with one only hypothesis test. However, in this study, we actually examined two main hypothesis tests: PP = Pp = pp and XX = Xx = xx in Chinese women. Using the cut-off of p < 0.05”for statistical significance of two hypothesis tests may increase the probability of false statistical significance. Thus, caution needs to be exercised in applying the marginally significant results in this study.
In conclusion, PvuII polymorphism had a very weak but marginally statistically significant association with femoral neck BMD, and PP homozygotes might have a lower BMD compared with p carriers. The XbaI polymorphism was unlikely to be associated with lumbar spine or femoral neck BMD in Chinese women.
References
Dalen N, Hellstrom LG, Jacobson B (1976) Bone mineral content and mechanical strength of the femoral neck. Acta Orthop Scand 47:503–508
Christian JC, Yu PL, Slemenda CW et al (1989) Heritability of bone mass: a longitudinal study in aging male twins. Am J Hum Genet 44:429–433
Pocock NA, Eisman JA, Hopper JL et al (1987) Genetic determinants of bone mass in adults. A twin study. J Clin Invest 80:706–710
Slemenda CW, Christian JC, Williams CJ et al (1991) Genetic determinants of bone mass in adult women: a reevaluation of the twin model and the potential importance of gene interaction on heritability estimates. J Bone Miner Res 6:561–567
Ioannidis J, Stavrou I, Trikalinos T et al (2002) Association of polymorphisms of the estrogen receptor alpha gene with bone mineral density and fracture risk in women: a meta-analysis. J Bone Miner Res 17:2048–2060
Ioannidis J, Ralston S, Bennett S et al (2004) Differential genetic effects of ESR1 gene polymorphisms on osteoporosis outcomes. JAMA 292:2105–2114
An XX, Chu WJ, Huang XY et al (2001) The study of the correlation between vitamin D; estrogen receptor gene polymorphisms and bone mineral density. Tianjin Med J (Chin) 29:451–454
Ge JR, Lin YP, Zhu XX et al (2004) Effect of Xba I polymorphisms of estrogen receptor gene and age on bone mineral density in postmenopausal women. Chin J Osteoporos (Chin) 10:439–442
Ge JR, Wang H, Zhu X et al (2006) Effect of Pvu II polymorphisms of estrogen receptor gene on filtering risk factors in postmenopausal osteoporosis. Chin J Osteoporos (Chin) 12:38–40
Guo S, Li D, Wang Q et al (2006) The effect of estrogen receptor gene on postmenopausal osteoporosis. Chinese General Practice (Chin) 9:200–203
Ho AY, Yeung SS, Kung AW (2000) PvuII polymorphisms of the estrogen receptor alpha and bone mineral density in healthy southern Chinese women. Calcif Tissue Int 66:405–408
Huang QR, Wang QH, Zhang LP et al (1998) Relation ship between bone mineral density and polymorphism of the estrogen receptor gene in postmenopausal health women. Chin J Osteoporos (Chin) 4:38–41
Lau EM, Young RP, Lam V et al (2001) Estrogen receptor gene polymorphism and bone mineral density in postmenopausal Chinese women. Bone 29:96–98
Li D, Cai X, Wu W et al (2006) Relationship between polymorphisms of estrogen receptor gene with bone mineral density in postmenopausal women. Int Med Health Guidance News (Chin) 11:4–5
Miao YX, Zhu HM, Zhu XY et al (2001) Relationship between bone mineral density and polymorphism of the estrogen receptor gene in the elderly in Shanghai. Geriatr Health Care (Chin) 7:225–227
Qin YJ, Zhang ZL, Huang QR et al (2004) Association of ER-1 gene PvuII and XbaI polymorphisms and related factors with osteoporosis in postmenopausal women. Chin J Geriatr (Chin) 23:380–383
Qin YJ, Zhang ZL, Huang QR et al (2004) Association of vitamin D receptor and estrogen receptor-alpha gene polymorphism with peak bone mass and bone size in Chinese women. Acta Pharmacol Sin 25:462–468
Wang YY, Li SY, Li KH et al (2004) Study on relationship between polymorphisms of estrogen receptor gene and female bone mineral density. Shanxi Med J (Chin) 33:478–480
Yang X, Zheng SR, Chen RJ et al (2004) The relationship between estrogen receptor allelic variants and peak bone mineral density in Beijing women. Chin J Clin Obstet Gynecol (Chin) 115:197–200
Zhang QX, Yang DZ, Kuang JQ et al (2001) Relationship between estrogen receptor genotypes and female bone mineral density. Chin J Obstet Gynecol (Chin) 36:344–347
Zhang XZ, Li Y (2002) Relationships of estrogen receptor gene polymorphism with bone metabolism in postmenopausal women. J Tongi Univ (Med Sci) (Chin) 23:1–4
Zhang YY, Long JR, Liu PY et al (2003) Estrogen receptor alpha and vitamin D receptor gene polymorphisms and bone mineral density: association study of healthy pre- and postmenopausal Chinese women. Biochem Biophys Res Commun 308:777–783
Chu WJ, An XX, Huang XY et al (2001) Relationship between bone mineral density and polymorphism of the estrogen receptor gene. Beijing Med J (Chin) 23:312–313
Huang Q, Wang Q, Zhang L et al (1999) Relationship between bone mineral density and polymorphism of the estrogen receptor gene in healthy postmenopausal women in China. Chinese Med J 112:832–835
Qin YJ, Zhang ZL, Huang QR et al (2003) Genetic, environmental and other factors and susceptibility of osteoporosis in postmenopausal women. Osteoporos Bone Miner Res (Chin) 2:70–75
Qin YJ, Shen H, Huang QR et al (2003) Estrogen receptor alpha gene polymorphisms and peak bone density in Chinese nuclear families. J Bone Miner Res 18:1028–1035
Wang QH, Huang QR, Zhou Q et al (2000) The relationship between bone mineral density and polymorphism of the estrogen receptor gene in Chinese healthy menopausal women. Chinese J Intern Med (Chin) 39:743–745
Wang YY, Wen XD, Li SY et al (2004) Study on Estrogen receptor and vitamin D receptor gene polymorphisms. Chinese J Gerontol (Chin) 24:888–889
Xue Y, Li D, Wang Q et al (2003) Pvu II polymorphisms of the estrogen receptor gene and bone mineral density in Beijing Han women. Beijing Med J (Chin) 25:6–9
Xue Y, Li D, Wang Q et al (2003) Xba I polymorphisms of the estrogen receptor gene and bone mineral density and BMI in Beijing Han women. Hereditas (Beijing) (Chin) 25:137–140
Xue Y, Wang Q, Li D et al (2003) Xba I polymorphisms of estrogen receptor gene and bone mineral density and BMI in Beijing Han women. Raumatol Orthop Q (Chin) 32:261–265
Xue Y, Li D, Yao L et al (2004) Relationship Between estrogen receptor gene polymorphism and osteoarthritis in Han women. Chin J Rheumatol (Chin) 8:583–586
Xue Y, Li D, Wang Q et al (2004) Effect of estrogen receptor gene polymorphism on the risk of osteoarthritis in Han women. Traumatol Orthop Q (Chin) 33:20–25
Xue Y, Li D, Yao L et al (2004) Relationship between estrogen receptor gene polymorphism and osteoarthritis in Han women. Osteoporos Bone Miner Res (Chin) 3:6–10
Yang X, Zheng SR, Chen RJ et al (2003) Study on the relationship between some genetic factors and peak bone mineral density in Beijing young women. Chin J Obstet Gynecol 38:273–276
Zhang XZ, Li Y, Qian GF et al (2004) Relationship of estrogen receptor gene polymorphism and bone metabolism and lipid metabolism in postmenopausal women. Prog Obstet Gynecol (Chin) 13:345–348
Zhang XZ, Li Y, Qian GF et al (2005) Relationship of estrogen receptor gene polymorphism and bone metabolism and lipid metabolism in post-menopausal women. Chin J Bone Tumor Bone Dis 4:229–233
An SJ, Li E, Tong XX et al (2000) Study on relationship between estrogen receptor gene polymorphism and syndrome differentiation typing of female postmenopausal osteoporosis in traditional Chinese medicine. Chin J Integrative Med (Chin) 20:907–910
An SJ, Cheng YR, Liu K et al (2001) Study on relationship between polymorphism of the estrogen receptor gene and bone mineral density, and bone metabolism. Chin J Orthop (Chin) 21:522–525
Liu JM, Zhu HM, Zhu XX et al (2001) The effect of estrogen receptor gene Px Haplotype on bone mineral density in Chinese postmenopausal women. Natl Med J China (Chin) 81:1295–1297
Liu JM, Zhu HM, Zhu XX et al (2002) Candidate genes of osteoporosis(ER, IL-6, COLIA1) in postmenopausal women. Chin J Osteoporos (Chin) 8:138–140
Liu J, Zhu H, Zhu X et al (2003) Estrogen receptor gene polymorphisms and bone mineral density in Chinese postmenopausal women. Chinese Med J 116:364–637
Liu JM, Zhu HM, Zhu XY et al (2003) Combined effect of interleukin-6 and estrogen receptor gene polymorphisms on bone mass in postmenopausal women. Chin J Obstet Gynecol (Chin) 38:24–27
Guan J, Dai ZH, Shen H et al (2000) Study on the association of estrogen receptor genotypes with bone mineral density in Chinese postmenopausal Han women in Beijing. J Beijing Med Univ (Chin) 32:508–511
Guan J, Dai ZH, Shen HT et al (2001) Study on the correlation of estrogen receptor gene polymorphism to bone mineral density of radius in Chinese Han postmenopausal women in Beijing. Chin J Obstet Gynecol (Chin) 36:40–42
Dvornyk V, Liu XH, Shen H et al (2003) Differentiation of Caucasians and Chinese at bone mass candidate genes: implication for ethnic difference of bone mass. Ann Hum Genet 67:216–227
Dvornyk V, Liu PY, Long JR et al (2005) Contribution of genotype and ethnicity to bone mineral density variation in Caucasians and Chinese: a test for five candidate genes for bone mass. Chin Med J (Engl) 118:1235–1244
Chen HY, Chen WC, Tsai HD et al (2001) Relation of the estrogen receptor alpha gene microsatellite polymorphism to bone mineral density and the susceptibility to osteoporosis in postmenopausal Chinese women in Taiwan. Maturitas 40:143–150
Lau HH, Ho AY, Luk KD et al (2002) Estrogen receptor beta gene polymorphisms are associated with higher bone mineral density in premenopausal, but not postmenopausal southern Chinese women. Bone 31:276–281
Dong YH, Liu L, Li CG et al (2002) Study on the relationship between estrogen receptor gene polymorphism and bone mineral density in diabetic patients. Chin J Endocrinol Metab (Chin) 18:214–218
Guan MP, Xue YM, Zhang Q et al (2003) Relationship between estrogen receptor gene polymorphism and bone mineral density in type 2 diabetic patients. Chin J Clin Relabilitation (Chin) 7:2140–2141
Yi GC, Shao ZT, Ruan B (2002) Polymorphisms of estrogen receptor gene distribution and its association with bone mineral density and replenishment calcium in pregnant women. Chinese J Birth Health Heredity (Chin) 10:16–17
Liu L, Dong YH, Si YG et al (2004) Relationship between polymorphism of ER gene and bone mass in type 2 diabetes mellitus. Chin J Osteoporos (Chin) 10:466–470
Liu YZ, Liu YJ, Recker RR et al (2003) Molecular studies of identification of genes for osteoporosis: the 2002 update. J Endocrinol 177:147–196
Thakkinstian A, McElduff P, D’Este C et al (2005) A method for meta-analysis of molecular association studies. Stat Med 24:1291–1306
van Houwelingen HC, Arends LR, Stijnen T (2002) Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 21:589–624
Long J, Liu P, Zhang Y et al (2003) Interaction effects between estrogen receptor alpha gene, vitamin D receptor gene, age, and sex on bone mineral density in Chinese. J Hum Genet 48:514–519
Colin EM, Uitterlinden AG, Meurs JBJ et al (2003) Interaction between vitamin D receptor genotype and estrogen receptor {alpha} genotype influences vertebral fracture risk. J Clin Endocrinol Metab 88:3777–3784
Kurabayashi T, Matsushita H, Kato N et al (2004) Effect of vitamin D receptor and estrogen receptor gene polymorphism on the relationship between dietary calcium and bone mineral density in Japanese women. J Bone Miner Metab 22:139–147
Mizunuma H, Hosoi T, Okano H et al (1997) Estrogen receptor gene polymorphism and bone mineral density at the lumbar spine of pre- and postmenopausal women. Bone 21:379–383
Deng HW, Li J, Li JL et al (1999) Association of VDR and estrogen receptor genotypes with bone mass in postmenopausal Caucasian women: different conclusions with different analyses and the implications. Osteoporos Int 9:499–507
Acknowledgement
The study was supported by the National Natural Science Foundation of China (Grant No. 30271120).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, CL., Tang, XY., Chen, WQ. et al. Association of estrogen receptor α gene polymorphisms with bone mineral density in Chinese women: a meta-analysis. Osteoporos Int 18, 295–305 (2007). https://doi.org/10.1007/s00198-006-0239-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-006-0239-2