Abstract
Aim
The effects of parathyroid hormone (PTH) alone or in combination with antiresorptive therapy on changes in bone mineral density (BMD) and fracture risk were studied.
Materials and methods
Randomised placebo controlled trials were retrieved from the PubMed, Web of Science or Embase databases.
Results
PTH alone or in combination with antiresorptive drugs reduced vertebral [relative risk (RR)=0.36, 95% confidence interval (CI): 0.28–0.47, 2p<0.01] and non-vertebral (RR=0.62, 95% CI: 0.48–0.82, 2p<0.01) fracture risk and increased spine BMD by 6.6% (95% CI: 5.2–8.1%, 2p<0.01) and hip BMD non-significantly by 1.0% (95% CI: −0.1 to 2.1%, 2p=0.08) during 11–36 months of follow-up (13 trials). The gain in spine and hip BMD tended to increase with the length of the PTH treatment. No significant effect of study duration on fracture risk could be demonstrated. The major adverse events were hypercalcaemia, nausea and discomfort at the injection sites. Only limited data are currently available on fracture risk reduction with PTH plus antiresorptive therapies.
Conclusion
Although the number of studies on non-vertebral fractures is limited, our pooled analysis revealed that PTH alone or in combination with antiresorptive drugs would appear to be able to reduce the risk of vertebral and non-vertebral fractures and to increase spine and perhaps hip BMD. However, these analyses were based on cross-sectional data – i.e. based on indirect comparisons – and further studies with a direct comparison of study duration are necessary. No studies comparing PTH, PTH plus antiresorptive drugs and antiresorptive drug versus placebo in a factorial design are available; consequently, we were unable to draw any conclusions on the superiority of PTH plus antiresorptive drug versus antiresorptive drug or PTH alone with respect to BMD or fractures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a major public health problem worldwide [1, 2]. However, during the past decade many new and highly efficient drug therapies have been introduced [3–8], most of which rely on antiresorptive principles. These include bisphosphonate therapy [9–11], estrogen and combined hormone replacement therapy (HRT) [12–14], selective estrogen receptor modulators (SERM) [12] and calcitonin [15], along with calcium and vitamin D [16–19]. More recently, strontium ranelate [3] has been added to the therapeutic options of an antiresorptive drug for osteoporosis.
Parathyroid hormone (PTH) [20] has come into use in clinical practice [5] as an anabolic principle [6, 20]. As more studies on both PTH(1-34) and PTH(1-84) are now becoming available, it is possible to analyse the effects of this drug in a meta-analysis. This is of particular interest as PTH may be used as monotherapy or in combinations with calcium and vitamin D, HRT [21], bisphosphonates [22–25] or nafarelin [26, 27]. However, many questions of PTH application in osteoporosis remain to be answered: (1) the optimal duration of PTH treatment, (2) the effects of PTH alone or combined with other treatment modalities [21, 22], (3) the effects of PTH dose and (4) the effects of different preparations of PTH [PTH(1-34) versus PTH(1-84)].
The question of duration of PTH therapy is especially interesting as the Neer and Orwoll studies [5] were terminated early due to reports on adverse events in rats. Available data from the studies suggest that a growth in bone mineral density (BMD) occurred beyond 18 months of treatment [28]; however, this growth rate may taper off, thereby making any extended continuation of treatment less attractive both clinically and cost-wise. On the other hand, there may be a continued growth in BMD after 18 months of such a magnitude that continuation of treatment would be interesting in terms of additional fracture prevention. In the meta-analysis reported here we addressed this question of treatment duration.
The objectives of this review were:
-
1)
Does BMD increase during treatment with PTH or PTH combined with antiresorptive drugs in comparison with placebo including calcium and vitamin D or antiresorptive drugs?
-
2)
Does PTH alone or in combination with antiresorptive drugs decrease the risk of both vertebral and non-vertebral fractures in comparison with placebo including calcium and vitamin D or antiresorptive drugs?
-
3)
Were there differences in the effects of PTH on BMD and fracture risk within the framework of duration of treatment, dose or type of PTH?
-
4)
What were the adverse effects of PTH treatment?
Materials and methods
We included randomised controlled trials with fracture occurrence or changes in BMD as the outcome and the use of PTH [all types, i.e. both PTH(1-34) and PTH(1-84)] as exposure. Inclusion criteria were randomised controlled studies with a duration of longer than 6 months where the active treatment arm of the study had to include PTH. Studies on both men and women ≥18 years of age were eligible along with studies in patients with secondary (for example, glucocorticoid-induced) osteoporosis. Studies comparing active drug plus, for example, alendronate compared to, for example, alendronate were also eligible. The placebo arm could include the use of calcium and vitamin D or other agents. Both abstracts and published papers were eligible. Exclusion criteria were non-randomised trials with a duration of less than 6 month and studies that did not report original data. Spine fractures had to be verified on X-rays. We also examined reference lists of retrieved studies for further relevant publications. If several publications reported on the same trial data, we chose the report with the longest follow-up, with one exception in the study by Black et al. [22, 23]. In the most recent of these two papers a pause of 12 months was introduced for PTH in one of the study arms [22]; to allow for an analysis of the effects of ongoing PTH, the report here focuses on the first paper [23]. A more detailed comparison of the papers is included in the Discussion. Two of the authors (PV and PS) reviewed the studies independently. We decided to study eligibility and data extraction by consensus. Interrater reliability was assessed, and no deviations in the studies retrieved. The quality of the studies was based on randomisation and placebo concealment of treatment using the Jadad scale [29]. The analyses were based on published papers only. No contact was made with lead authors or industrial companies.
The rationale for choosing all studies of PTH, irrespective of whether they were on pre- or postmenopausal osteoporosis, were performed in men or women or used PTH(1-34) or PTH(1-84), was to assess the effect of PTH on fracture risk or BMD under all possible conditions. If studies were excluded – for whatever the reason – it would not be possible to assess heterogeneity due to differences in, for example, PTH type or gender. Given the design of the meta-analysis, the absence of statistical heterogeneity – despite clinical heterogeneity – would indicate a universal reproducible effect of PTH.
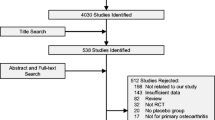
The search date was November 1, 2005. We retrieved studies from PubMed (1951 and onwards), Embase (1974 and onwards), Science Citation Index (1945 and onwards), the Cochrane Central Register of Controlled trials and conference abstracts from the Journal of Bone Mineral Research, Calcified Tissue International and Osteoporosis International from 1990 onwards. Reference lists of papers selected along with reference lists from review papers were also screened for studies of interest. The search was two-pronged to ensure that both studies with fractures and BMD as endpoint were included. In the first search we used the terms “parathyroid hormone” and “fracture” with the limits “human” and “randomised controlled trial”, and this search produced 56 trials from all of the databases searched. A second search using the MESH terms “parathyroid hormone” and “bone mineral” with the limits “human” and “randomised controlled trial” produced 200 trials. The two searches combined produced 214 studies. Among these 214 studies, 201 either did not include PTH treatment (but rather measurements of serum PTH), were not randomised controlled trials, were reviews, did not report original data or reported data or results from other trials of PTH that were already included in our search (Fig. 1, Table 1).
There was a duplication of patients between Cosman et al. [24] and Lindsay et al. [21], with the latter study reporting preliminary results from a subgroup and the first reporting the full data set.
The data extracted consisted of number of patients with one or more vertebral or non-vertebral fractures during follow-up, lumbar spine and hip areal BMD. Only two of 11 studies with data on hip BMD provided data on total hip [24, 25] BMD and not only femoral neck BMD (128 participants out of a total of 2886). Exclusion of these studies did not change the results. Data on forearm BMD were not included, as only eight of the 12 studies provided data on forearm BMD, with differences between the studies in the region of the radius analysed: five provided data on radius 1/3, two provided data on distal radius and one provided data on radius shaft.
Serious adverse events were defined as clinical events for the patients, and changes in serum measurements without clinical implications for the patient were not included in the table. Data on adverse events were based on adverse events reported by the studies (Table 2). The adverse events were categorised by frequency with which they occurred.
Testing for clinical heterogeneity was based on gender (men vs. women), age (>70 vs. ≤70 years at the start of the study), study duration (≤18 vs. >18 month), drug combination used [PTH(1-34) or PTH(1-84)], alone or in combination with HRT, bisphosphonate or nafarelin), dose of drug used [20–40 μg per day of PTH(1-34) and 25–100 μg per day of PTH(1-84)]. Exclusion of the studies on men or on corticosteroid-induced osteoporosis did not change the results.
Statistics
We calculated a weighted risk estimate for binary outcomes such as fracture based on relative risks (RR) calculated from the crude data in the studies. A χ2 test for heterogeneity was applied. We chose a random effects model for heterogeneous outcomes, and a fixed effects model for homogeneous outcomes [30]. In the case of homogeneity, the results were not affected by a shift to the random effects models (data not shown). For continuous outcomes, such as BMD, we calculated the weighted mean difference (WMD) both for Z-score changes and for percentage changes in BMD. We performed tests for heterogeneity using funnel plots [30]. Analyses of potential publication bias were also performed. We used Review Manager 4.2.7 from the Cochrane Collaboration for statistical analyses http://www.cc-ims.net/RevMan/download.htm. In some cases the number of fractures was zero in one of the groups. In such cases the programme of the Cochrane Collaboration calculated the RR according to international standards. For the adverse events, Peto Odds ratios were also calculated.
RRs were compared directly: following logarithmic transformation, the numbers were then compared by the t-test. This is in effect the same manner in which mean values are compared using the 95% confidence intervals (95% CI) calculated from the standard error of the mean (mean of sample 1±SE of sample 1 vs. mean of sample 2±SE of sample 2). This allowed comparison of the RR for one treatment with that of another. However, one should be aware that this represents an indirect method [31].
For the comparison of study duration versus change in BMD the fit with various functions (linear, logarithmic, polynomial) was examined, and the curve with the best fit (highest correlation coefficient) was chosen.
Results
Table 1 shows baseline characteristics of the 13 studies on PTH. Concomitant treatment with calcium and/vitamin D was given in 12 studies, estrogen in two studies, bisphosphonate (alendronate) in five studies and nafarelin in one study.
Effects on fracture risk
Table 3 shows the effects of PTH alone or in combination. PTH alone or in combination with antiresorptive drugs reduced vertebral fracture risk by 64% (RR=0.36, 95% CI: 0.28–0.47, 2p<0.01; seven studies, n=4359) and non-vertebral fracture risk by 38% (RR=0.62, 95% CI: 0.48–0.82, 2p<0.01; five studies, n=2377). We found no heterogeneity among the studies.
For PTH alone or in combination with antiresorptive drugs the reduction in vertebral fractures (64%) was significantly larger than the reduction in non-vertebral fractures (38%, 2p<0.01) by direct comparison of the estimates.
The large studies by Neer et al. [5] and Greenspan et al. [20] contributed the most patients to this meta-analysis. A sensitivity analysis excluding the studies by Neer et al. [5], Orwoll et al. [6] and Greenspan et al. [20] therefore did not change the results. The RR for vertebral fractures was 0.37 (95% CI 0.14–0.98, 2p<0.01 for the small studies combined; four studies, n=222).
Funnel plots did not reveal signs of publication bias.
Subgroup analyses on fracture risk
Effects of duration of treatment with PTH on fracture risk
The fracture risk reduction for vertebral fractures during PTH treatment alone or in combination did not change with study duration. For a therapy of ≤18 month duration, the relative risk was 0.43 (95% CI: 0.29–0.65, 2p<0.01; five studies, n=2981), and for a therapy >18 month in duration the relative risk was 0.32 (95% CI: 0.23–0.45, 2p<0.01; two studies, n=1378). By direct comparison of the estimates, there was a non-significant trend towards a decrease (p=0.13). For non-vertebral fractures, the figures for a therapy of ≤18 month were RR=0.65, 95% CI: 0.33–1.30 (four studies, n=740) and for a therapy of >18 month, RR=0.62, 95% CI: 0.46–0.83 (only the Neer study) – i.e. no difference.
Effects on fracture risk of PTH alone and PTH combined with other drugs
The effect of PTH alone versus placebo on vertebral fractures (RR=0.37, 95% CI: 0.28–0.48, 2p<0.01; four studies, n=4155) was within the range found for PTH+HRT (RR=0.12, 95% CI: 0.01–0.99, 2p=0.68; two studies, n=96, ). This difference was not statistically significant (2p=0.34) by a direct comparison of the estimates. For non-vertebral fractures, only two studies (Neer et al. [5] and Orwoll et al. [6]) compared PTH alone with placebo. The combined estimate of these two studies was RR=0.62, 95% CI: 0.46–0.82, 2p<0.01.
Effects of dose of PTH and PTH type [PTH(1-34) versus PTH(1-84)]
The effect of 100 μg PTH(1-84) on vertebral fracture risk [RR=0.39, 95% CI 0.22–0.69, 2p<0.01; study by Greenspan et al. (20)] was in the same range as the effect of PTH(1-34) (20 μg: RR=0.31, 95% CI 0.19–0.50, 2p<0.01; 40 μg: RR=0.35, 95% CI 0.22–0.55, 2p<0.01; study by Neer et al. [5], 2p=0.27 and 2p=0.39, respectively, for direct comparison of estimates).
Effects on BMD
Table 4 shows the effects of PTH on BMD in the spine and hip. PTH increased spine BMD by 6.6% (95% CI: 5.2–8.1%, 2p<0.01; 12 studies, n=3080) and hip BMD non-significantly by 1.0% (95% CI: −0.1 to 2.1%, 2p=0.08; 11 studies, n=2886) during 11–36 months of follow-up. The changes in BMD displayed heterogeneity. Funnel plots did not reveal signs of publication bias. A sensitivity analysis excluding the large studies of Neer et al. [5] and Orwoll et al. [6] showed an increase in spine BMD Z-score of 0.37 (95% CI 0.24–0.50, 2p<0.01; nine studies, n=1144) and in hip BMD Z-score of 0.01 (95% CI: −0.02 to 0.04, 2p=0.51; nine studies, n=1009) – i.e. close to that when the large studies were included for the spine, but lower in the hip.
Subgroup analyses on BMD
Effects of duration of treatment with PTH on BMD
The effect of PTH on spine BMD Z-score correlated significantly with the duration of the treatment based on cross-sectional BMD data at the termination of the study (mean increase ± SE: ≤18 months: 0.37, 95% CI: 0.24–0.50, 2p<0.01; nine studies, n=1456; >18 months: 0.75, 95% CI: 0.56–0.94, 2p<0.01 SD; three studies, n=1624; 2p<0.01 for comparison). These estimates also displayed heterogeneity for ≤18 month (p<0.01) and for >18 month (p<0.01).
In the hip the trend was similar based on cross-sectional BMD data at the termination of the studies, with a larger increase with >18 months of treatment than with ≤18 months of treatment (≤18 months: 0.01, 95% CI: −0.01 to 0.04, 2p=0.43; eight studies, n=1321; >18 months: 0.21, 95% CI: 0.09–0.33, 2p<0.01; three studies, n=1565; 2p<0.01 for comparison). These estimates also displayed heterogeneity for ≤18 month (p<0.01) and for >18 month (p<0.01).
Figure 2 shows the percentage net increase in the spine and hip.
Percentage net increase in bone mineral density (BMD; treated minus control – i.e. weighted mean difference) as a function of time. The best fit was obtained for a logarithmic curve (function inserted). R 2, squared correlation coefficient. Note that the analysis is based on cross-sectional data from the time of termination of the study and not on true longitudinal data. a Spine, b hip
Effects on BMD of PTH alone and PTH combined with other drugs
The increase in the spine BMD Z-score following treatment with PTH alone in comparison to treatment with a placebo was 0.51 (95% CI: 0.30–0.72, 2p<0.01; four studies, n=2162); in the hip the increase was 0.11 (95% CI: 0.04–0.18, 2p<0.01; four studies, n=2103). These estimates were heterogeneous for the spine (p<0.01) and for the hip (p<0.01).
With PTH alone or in combination with alendronate versus alendronate alone the increase in spine BMD Z-score was 0.28 (95% CI: 0.16–0.40, 2p<0.01; five studies, n=783. This estimate was heterogeneous (p<0.01). The increase in the spine BMD Z-score was borderline significantly lower than that for PTH versus the placebo (2p=0.06). In the hip the increase in the Z-score with PTH alone or in combination with alendronate compared to alendronate was 0.00 (95% CI: −0.04 to 0.05, 2p=1.00; four studies, n=640). This estimate did not display heterogeneity (p=0.56). The increase in the hip BMD Z-score was significantly lower than for that for PTH versus the placebo (2p<0.01).
The mean increase in spine BMD Z-score was 0.80 (95% CI: 0.50–1.09, 2p<0.01) with PTH plus HRT, but only two studies were available (n=92). This increase in spine Z-score with PTH+HRT was similar to that observed with PTH compared to the placebo (2p=0.12) but higher than that seen with PTH alone or in combination with alendronate (2p<0.01). In the hip the increase in BMD Z-score with PTH plus HRT was 0.16 (95% CI: −0.06 to 0.38, 2p=0.15), but only two studies were available (n=100). This increase was similar to that seen with PTH compared to the placebo (2p=0.67) and PTH alone or in combination with alendronate versus alendronate (2p=0.16).
On the other hand, the mean duration of studies differed. For PTH versus the placebo the mean duration of the treatment 18 months (95% CI: 14–21 months), and for PTH plus alendronate it was 14 months (95% CI: 0–28 months). For the two studies on PTH+HRT, the mean study duration was 24 months (range: 12–36 months). Adjustment for the duration of the study showed that the effect of treatment type was still present (2p<0.01).
Effects of dose of PTH and PTH type [PTH(1-34) versus PTH(1-84)]
The effects of 20–40 μg PTH(1-34) compared to those of the placebo (BMD Z-score: 0.67, 95% CI: 0.46–0.88, 2p<0.01 in the spine; BMD Z-score: 0.17, 95% CI; 0.04–0.29, 2p<0.01 in the hip). Neer et al. [5] and Orwoll et al. [6]) were a little higher for 50–100 μg PTH(1-84) (BMD Z-score: 0.28, 95% CI: 0.15–0.41, 2p<0.01 in the spine; BMD Z-score: 0.01, 95% CI −0.02 to 0.04, 2p=0.51 in the hip; Hodsman et al. [32] and Kurland et al. [33]), but the comparison was severely hampered by differences in study duration: 11 and 21 months in the Orwoll [6] and Neer study [5] and 12 and 18 months in the Hodsman [32] and Kurland study [33], with the Neer study [5] by far being the largest. A direct comparison was thus not possible due to the low number of studies and the differences in study characteristics.
The study by Hodsman et al. [32] showed a trend towards higher BMD with higher PTH(1-84) dose, and a similar trend for 40 versus 20 μg of PTH(1-34) was present in the Neer [5] and Orwoll study [6].
Serious adverse effects
Table 2 summarizes studies reporting serious adverse events associated with use of PTH. Headache was reported by two studies to be more frequent with 40 μg PTH(1-34) than with 20 μg PTH or placebo [5, 6], but the numbers were only partially reported in one of the studies [6]. One study reported more headache with PTH(1-84) and PTH(1-84) in combination with alendronate than with alendronate alone [28]. Dizziness was reported by one study to be more frequent with 20 μg PTH(1-34) than with 40 μg PTH or with placebo [5], and another study reported more dizziness with PTH(1-84) alone or in combination with alendronate than with alendronate alone. Redness and pain at the injection sites were reported in many trials, but the main difference was seen in studies where no placebo injections were administered. It was not possible to perform statistical analyses of the differences in side effects between PTH(1-34) and PTH(1-84) as most available studies reported data on PTH(1-34).
For the following events studies were numerous enough to allow statistical analysis.
Back pain
Four studies reported on the frequency of back pain (Table 2). One additional study not included in the meta-analysis also reported on back pain with 20 μg PTH(1-34) [34].
The pooled analysis showed a significant reduction in patients with new or worsening back pain with PTH (Peto Odds ratio: OR=0.68, 95% CI: 0.53–0.87; five studies, n=1366). The treatment results were not heterogeneous (χ2=7.96, df=4, p=0.09).
Nausea
Three studies reported on the frequency of nausea (Table 2). The Peto Odds ratio for nausea was 1.89 (95% CI: 1.47–2.42 for PTH vs. other treatments; four trials, n=2199). There was significant heterogeneity (χ2=15.83, df=3, p<0.01). The two studies reporting on the PTH(1-34) 40 μg dose reported a higher Peto Odds ratio of 2.71 (95% CI: 1.97–3.72) than the studies on lower doses (Peto Odds ratio: 1.08, 95% CI: 0.72–1.60, 2p<0.01 by direct comparison).
Hypercalcaemia
The occurrence of hypercalcaemia was very dependent on the timing of the measurements after the PTH injections, with most cases of hypercalcaemia occurring 4–6 h following the injection with a decrease in incidence with time.
If one looks at all reports of elevated serum calcium at any time during the follow-up (irrespective of degree of elevation) then 21.8% (95% CI: 20.3–23.3%) of the PTH-treated groups developed hypercalcaemia in comparison to 3.0% (95% CI: 2.3–3.6%) in the non-PTH-treated groups. The weighted risk difference was 11% (95% CI: 4–18%). The estimate was highly heterogeneous (ten studies, n= 4872, χ2=207, df=11, p<0.01). The Peto Odds ratio of hypercalcaemia was 5.82 (95% CI: 4.96–6.84).
Discussion
The present systematic review revealed that therapy with PTH alone or in combination with other therapies increased spine and hip BMD and significantly reduced the risk of vertebral as well as non-vertebral fractures – although the number of studies on non-vertebral fractures was limited. It also disclosed that the effects on BMD seemed to be intensified by a treatment time of more than 18 months. No significant effect of treatment duration on fracture risk could be demonstrated. It should also be emphasised that the effect of time on BMD and fracture risk was based on indirect comparisons and analyses of cross-sectional data obtained at the time of termination of the studies and that further studies based on longitudinal data with a direct comparison of different treatment durations are needed.
A large clinical PTH study [5] was interrupted after 21 months due to the finding of an increased risk of osteosarcoma among a specific strain of rats treated with very large doses of PTH(1-34). Since that time, the treatment time for PTH therapy in most countries is limited to 18–24 months. From Fig. 2 one can calculate the theoretical gain in BMD if the treatment duration were to be increased from 18 to 24 months. In the spine the theoretical gain would be approximately 1.6% higher after 24 months (9.3%) than after 18 months (7.7%); in the hip the theoretically expected BMD gain would be approximately 0.6% larger (2.0 vs. 1.3%). However, further studies are needed to confirm if the increase in BMD is actually reflected in a decrease in fracture risk. When interpreting these data one should also realise that an extension of treatment is also accompanied by higher costs and, perhaps, more side effects. The safety of longer treatment with PTH also remains to be established, although the Neer study [5] failed to show an excess of serious adverse events, including deaths and cancer, after an average of 21 months of therapy, with the exception of differences in the number of episodes of hypercalcaemia, pain at injection sites, headache and nausea. An cost-effectiveness analysis revealed that PTH(1-34) treatment in an 69-year-old woman with a femoral neck T-score of −3 had a price tag per QUALY of 20,000 euro if the woman had had a recent spine fracture [35], which may be in a range that suggests moderate evidence to adopt such a therapy [36]. More recent cost-effective analyses have attempted to compare different therapies for osteoporosis [37]. However, further detailed analyses have to be made on the cost effectiveness of extending the duration of PTH therapy.
The fracture risk estimates did not display heterogeneity despite differences in treatment duration and treatment mode, but the number of studies was limited. The BMD results displayed a large degree of heterogeneity. However, despite the stratification, significant differences were still present with respect to dose of PTH, dose and type of antiresorptive drug, administration form of PTH (continuously or intermittently) and whether PTH was compared to placebo (calcium plus vitamin D) or antiresorptive drug.
The analyses in this paper are based both on PTH therapy alone compared to placebo and PTH therapy in combination with antiresorptive drugs. One should therefore not confuse the effect of PTH alone with that of PTH in combination with antiresorptive drugs. Further studies on the combination therapies are needed. Previous meta-analyses have suggested a discrepancy between change in BMD and change in fracture risk with antiosteoporotic treatment [38, 39]. In this context, fracture risk reduction is the clinically relevant end-point. A large reduction in spine fracture risk was observed with a smaller but still significant reduction in non-vertebral fracture risk. It remains to be shown whether a statistically significant reduction in hip fracture risk can be achieved with PTH therapy. As new and effective treatments for osteoporosis are coming onto the market, it would not be ethical to hold a new placebo-controlled trial on PTH. A possible alternative would be that a new study compare, for example, PTH for 18 or 24 months followed by 18 or 12 months of a bisphosphonate with a bisphosphonate for the entire 36-month period.
In our study HRT plus PTH showed similar gains in BMD as PTH therapy alone, but the gain in BMD was higher with the former than with PTH in various combinations with alendronate. However, this should not be over-interpreted: in these studies, PTH is an add-on to the other therapy. One possibility for the difference between HRT and alendronate is that alendronate is a more persistent inhibitor of bone turnover than HRT. PTH is thus better able to increase bone formation in combination with HRT than with alendronate. However, the number of studies was small. After 1 year, PTH alone, PTH+alendronate and alendronate alone produced similar increases in areal BMD in the spine [23]. In the hip PTH+alendronate and alendronate alone produced greater increases than PTH alone in areal BMD [23]. However, trabecular volumetric BMD increased significantly more in both the spine and hip with PTH alone than with PTH+alendronate or alendronate alone [23]. A follow-up of this study showed that discontinuation of PTH led to a decline in areal spine BMD, PTH followed by alendronate led to a continued increase in BMD, while PTH+alendronate produced an increase in areal spine BMD that was comparable to that of alendronate alone [22]. PTH followed by a bisphosphonate – for example, alendronate – is thus a beneficial therapeutical principle. However, our meta-analysis suggests that an extension of PTH treatment from 18 to 24 month-years would lead to an even greater increase in BMD that could then be substantiated by follow-up therapy with a bisphosphonate.
There was a dose response relationship for BMD with both PTH(1-34) and PTH(1-84). However, the cost effectiveness of this remains to be proven as no difference in fracture risk could be demonstrated between the 20 and 40 μg PTH(1-34) dose.
In the present analyses most of the data originated from trials on PTH(1-34), whereas the amount of data currently available on PTH(1-84) is more limited. More data are thus needed on PTH(1-84) to establish if any clinically significant differences actually do exist between PTH(1-34) and PTH(1-84) in terms of osteoporosis management. The direct comparison of spine fracture risk between PTH(1-34) and PTH(1-84) may have been hampered by differences in the study populations. A study with a direct comparison of these two agents is thus needed to reveal any (possible) differences.
We found that the risk reduction with PTH alone or in combination with antiresorptive therapies was 64% for vertebral fractures and 38% for non-vertebral fractures. For the bisphosphonates risk reductions of 48 and 49% for vertebral and non-vertebral fractures, respectively, was seen for alendronate [10], and 36 and 27% with risedronate [11]. For raloxifene risk reductions of 40 and 8% have been reported for vertebral and non-vertebral fracture risk, respectively [12]. However, direct comparisons of these estimates are severely hampered by differences in inclusion criteria (prevalent spine fractures or risk factors), dose of drug, among others [40].
Similar to other meta-analyses our study has some limitations. First, the analysis is only based on published data, and no unpublished data are included. Furthermore, heterogeneity in the patient cohorts with respect to age and ethnic origin should be expected, and it is therefore impossible to match the cohorts completely in the analysis. On the other hand, this heterogeneity may be of benefit as the results are representative for the effects on a broader population. Differences in treatment length and design as well as in the severity of disease of the participants are also factors that potentially introduce difficulties in the analysis, as large differences in treatment effects can be expected. Previous fracture is an independent predictor of future fractures that is largely independent of BMD [41]. In studies that include patients with previous fractures the absolute risk of incident fractures would be expected to be higher than that in studies with patients without prior fractures. In patients with a high absolute risk of fractures it may be easier to show significance rather than to compare their absolute risk of fracture with patients with a lower fracture risk. Among the PTH studies, several studies were based on patients with prior fractures, and this may have influenced the fact that the treatment effect was statistically significant. In this meta-analysis we have relied on published aggregated data. Another approach to a meta-analysis is to obtain individual patient data and analyse these. Each method of approach has its own advantages and disadvantages. In the analysis of the interaction with treatment duration, individual data may not have provided an advantage, as data would have to be analysed with time-dependent covariates, and the exact date of a spine fracture cannot always be determined.
Finally only five of the eligible studies were designed to evaluate non-vertebral fractures, which reduces the power the this meta-analysis. Data are also is lacking on specific skeletal sites of osteoporotic fractures, such as the hip and forearm.
We conclude that PTH alone or in combination with antiresorptive drugs reduces vertebral and non-vertebral fracture risk, although the number of studies on non-vertebral fractures is limited. The gain in spine and hip BMD seemed to be larger in patients treated with PTH for more than 18 months than in those treated for a shorter period of time. However, further studies are needed to corroborate the positive effect of increasing the duration of PTH treatment on outcome as the results in this analysis are based on indirect comparisons. To date there are no studies available that compare PTH, PTH plus antiresorptive drugs and antiresorptive drug versus placebo in a factorial design. Consequently, we are unable to draw any conclusions on the (possible) superiority of PTH plus antiresorptive drug versus antiresorptive drug or PTH alone in the context of BMD or fractures.
References
Johnell O (1997) The socioeconomic burden of fractures: today and in the 21st century. Am J Med 103:20S–25S
Kannus P, Niemi S, Parkkari J et al (1999) Hip fractures in Finland between 1970 and 1997 and predictions for the future (see comments). Lancet 353:802–805
Meunier PJ, Slosman DO, Delmas PD et al (2002) Strontium ranelate: dose-dependent effects in established postmenopausal vertebral osteoporosis – a 2-year randomized placebo controlled trial. J Clin Endocrinol Metab 87:2060–2066
Meunier PJ, Roux C, Seeman E et al (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350:459–468
Neer RM, Arnaud CD, Zanchetta JR et al (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Orwoll ES, Scheele WH, Paul S et al (2003) The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18:9–17
Reginster JY, Meunier PJ (2003) Strontium ranelate phase 2 dose-ranging studies: PREVOS and STRATOS studies. Osteoporos Int 14:56–65
Reginster JY, Seeman E, de Vernejoul MC et al (2005) Strontium ranelate reduces the risk of non-vertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) Study. J Clin Endocrinol Metab 90:2816–2822
Cranney A, Guyatt G, Krolicki N et al (2001) A meta-analysis of etidronate for the treatment of postmenopausal osteoporosis. Osteoporos Int 12:140–151
Cranney A, Wells G, Willan A et al (2002) Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev 23:508–516
Cranney A, Tugwell P, Adachi J et al (2002) Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endocr Rev 23:517–523
Cranney A, Tugwell P, Zytaruk N et al (2002) Meta-analysis of raloxifene for the prevention and treatment of postmenopausal osteoporosis. Endocr Rev 23:524–528
Torgerson DJ, Bell-Syer SEM (2001) Hormone replacement therapy and prevention of non-vertebral fractures. A meta-analysis of randomized trials. JAMA 285:2891–2897
Torgerson DJ, Bell-Syer SEM (2001) Hormone replacement therapy and prevention of vertebral fractures: a meta-analysis of randomised trials. BMC Musculoskelet Disord 2:7–10
Cranney A, Tugwell P, Zytaruk N et al (2002) Meta-analysis of calcitonin for the treatment of postmenopausal osteoporosis. Endocr Rev 23:540–551
Papadimitropoulos E, Wells G, Shea B et al (2002) Meta-analysis of the efficacy of vitamin D treatment in preventing osteoporosis in postmenopausal women. Endocr Rev 23:560–569
Shea B, Wells G, Cranney A et al (2002) Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis. Endocr Rev 23:552–559
Shea B, Wells G, Cranney A et al (2004) Calcium supplementation on bone loss in postmenopausal women (Review). Cochrane Database Syst Rev CD004526
Bischoff-Ferrari HA, Willett WC, Wong JB et al (2005) Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 293:2257–2264
Greenspan SL, Bone HG, Marriott TB et al (2005)Preventing the first vertebral fracture in postmenopausal women with low bone mass using PTH(1–84): results from the TOP study (Abstract). J Bone Miner Res 20:S56
Lindsay R, Nieves J, Formica C et al (1997) Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 350:550–555
Black DM, Bilezikian JP, Ensrud KE et al (2005) One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med 353:555–565
Black DM, Greenspan SL, Ensrud KE et al (2003) The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215
Cosman F, Nieves J, Woelfert L et al (2001) Parathyroid hormone added to established hormone therapy: effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res 16:925–931
Cosman F, Nieves J, Zion M et al (2005) Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med 353:566–575
Finkelstein JS, Klibanski A, Arnold AL et al (1998) Prevention of estrogen deficiency-related bone loss with human parathyroid hormone(1-34). JAMA 280:1067–1073
Finkelstein JS, Arnold AL (1999) Increases in bone mineral density after discontinuation of daily human parathyroid hormone and gonadotropin-releasing hormone analog administration in women with endometriosis. J Clin Endocrinol Metab 84:1214–1219
Finkelstein JS, Hayes A, Hunzelman JL et al (2003) The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 349:1216–1226
Jadad AR, Moore RA, Carroll D et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Böhning D (2000) Computer-assisted analysis of mixtures and applications: meta-analysis, disease mapping and others. Chapman&Hall/CRC, Boca Raton
Bucher HC, Guyatt GH, Griffith LE, Walter SD (1997) The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 50:683–691
Hodsman AB, Hanley DA, Ettinger MP et al (2003) Efficacy and safety of human parathyroid hormone-(1-84) in increasing bone mineral density in postmenopausal osteoporosis. J Clin Endocrinol Metab 88:5212–5220
Kurland ES, Cosman F, McMahon DJ et al (2000) Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab 85:3069–3076
Genant HK, Halse J, Briney WG et al (2005) The effects of teriparatide on the incidence of back pain in postmenopausal women with osteoporosis. Curr Med Res Opin 21:1027–1034
Lundkvist J, Johnell O, Cooper C, Sykes D (2006) Economic evaluation of parathyroid hormone (PTH) in the treatment of osteoporosis in postmenopausal women. Osteoporos Int 17:201–211
Rosner AJ, Grima DT, Torrance GW et al (1998) Cost effectiveness of multi-therapy treatment strategies in the prevention of vertebral fractures in postmenopausal women with osteoporosis. Pharmacoeconomics 14:559–573
Liu H, Michaud K, Nayak S et al (2006) The cost-effectiveness of therapy with teriparatide and alendronate in women with severe osteoporosis. Arch Intern Med 166:1209–1217
Delmas PD, Li Z, Cooper C (2004) Relationship between changes in bone mineral density and fracture risk reduction with antiresorptive drugs: some issues with meta-analyses. J Bone Miner Res 19:330–337
Delmas PD, Seeman E (2004) Changes in bone mineral density explain little of the reduction in vertebral or non-vertebral fracture risk with anti-resorptive therapy. Bone 34:599–604
Wehren LE, Hosking D, Hochberg MC (2004) Putting evidence-based medicine into clinical practice: comparing anti-resorptive agents for the treatment of osteoporosis. Curr Med Res Opin 20:525–531
Kanis JA, Johnell O, De Laet C et al (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35:375–382
Lane NE, Sanchez S, Modin GW et al (1998) Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest 102:1627–1633
Body JJ, Gaich GA, Scheele WH et al (2002) A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1–34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 87:4528–4535
McClung MR, San Martin J, Miller PD et al (2005) Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 165:1762–1768
Acknowledgement
Research librarian ms. Edith Clausen is acknowledged for skillful help with the references.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vestergaard, P., Jorgensen, N.R., Mosekilde, L. et al. Effects of parathyroid hormone alone or in combination with antiresorptive therapy on bone mineral density and fracture risk – a meta-analysis. Osteoporos Int 18, 45–57 (2007). https://doi.org/10.1007/s00198-006-0204-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-006-0204-0