Abstract
This study aimed to evaluate the effects of teriparatide [hPTH (1–34)] on quantitative ultrasound (QUS) parameters and bone mineral density (BMD) at the axial and appendicular (hand) skeleton in women with established osteoporosis who had been previously treated with antiresorptive drugs. Sixty postmenopausal women (age 71.1±6.8 years) were randomly assigned to either receive once-daily 20-μg subcutaneous teriparatide (n=30) or continue the antiresorptive treatment (n=30). At baseline and at 2-month intervals we measured QUS parameters at the calcaneus using the Achilles Plus (GE, Lunar), measuring speed of sound (SOS), broadband ultrasound attenuation (BUA), and stiffness index; QUS parameters at the phalanxes using the Bone Profiler (IGEA), measuring amplitude-dependent speed of sound (AD-SoS), bone transmission time (BTT), and fast wave amplitude (FWA); and BMD values at the right hand using dual x-ray absorptiometry. BMD at the lumbar spine, femur, and whole body were measured on a 6-monthly basis. After 1 year of teriparatide treatment, the changes in BMD were 7.1% at the lumbar spine, 2.6% at the femoral neck, −0.8% at the total hip, and −0.6% for the whole body. Teriparatide induced a significant and persistent decrease in BMD at the hand (−3.6% at month 6 and −2.7% at month 12). In the teriparatide group at month 12, AD-SoS was slightly increased (0.7%; not significant), whereas BTT significantly decreased (−16.4%, p<0.001) and FWA significantly increased (17.5%, p<0.001). The FWA/BTT ratio increased by 26.6% and 32.9% at months 6 and 12, respectively, in the teriparatide group and remained unchanged in the antiresorptive group. In women with established osteoporosis who had previously been treated with various antiresorptive drugs, 1 year of teriparatide treatment determined the expected increase in BMD at the axial skeleton and a significant and prolonged decrease in BMD at the hand. Moreover, teriparatide determined important changes in BTT and FWA, two parameters obtained from the analysis of ultrasonographic trace at the phalanxes, which could be considered in monitoring for the early effect of teriparatide on bone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The currently accepted treatment of postmenopausal osteoporosis uses bisphosphonates, selective estrogen-receptor modulators, and salmon calcitonin. These drugs all act by reducing bone turnover, which is usually associated with an increase in bone mineral density (BMD) and a lower risk of subsequent fragility fractures [1].
However, even with the use of such antiresorptive treatment, many patients continue to have a BMD that remains within the osteoporotic range, and many continue to have fractures. Therefore, there is a great need for new classes of drugs. With the approval of teriparatide [hPTH (1–34)], a potent anabolic drug has been added to the therapies currently available for osteoporosis. In fact, teriparatide increases BMD by stimulating bone formation rather than by reducing bone resorption [2–4]. In a placebo-controlled clinical trial, once-daily administration of teriparatide for a median of 19 months significantly increased BMD at most skeletal sites and substantially reduced the risk of vertebral and nonvertebral fractures [5].
However, the observed decrease in BMD at the distal radius in the study by Neer et al. [5] seemed to stimulate the hypothesis of a negative effect of teriparatide on skeletal sites made up predominantly of cortical bone. This doubt has been overcome by the finding that teriparatide determines a striking decrease in fracture risk at the appendicular skeleton [5]. Moreover, by using quantitative computed tomography (QCT), two recent studies have demonstrated that teriparatide increases cortical thickness and cross-sectional area at both the axial and appendicular skeleton [6, 7]. Moreover, many animal and human studies have reported that teriparatide greatly improves bone quality (microarchitecture, trabecular connectivity, geometry, etc.) and bone strength [8–10]. Therefore, there is a growing conviction that BMD measurement by dual x-ray absorptiometry (DXA) is unable to register all the positive changes induced in bone by teriparatide; in particular, the measurement of BMD by DXA seems to underestimate the amount of new bone made by teriparatide until secondary mineralization is concluded [11, 12]. The last decade has seen growing interest in the assessment of bone status using quantitative ultrasound (QUS) techniques, which may offer an attractive possible alternative to the central DXA assessment because they are radiation-free, relatively cheap, and easily transportable [13]. Moreover, many studies have reported that QUS parameters may reflect not only bone density but also other qualitative properties of bone that are strictly related to bone strength (elasticity, structure, microarchitecture) [14].
Several studies carried out in postmenopausal women [15, 16] and elderly men [17, 18] have demonstrated that QUS can predict fragility fracture risk independent of BMD. In a previous study we reported that patients with primary hyperparathyroidism presented a pattern of QUS parameters—obtained from graphic trace analysis of the ultrasonographic signal at phalanxes—that was characteristic and different from that of postmenopausal osteoporosis [19]. Some studies have found that in postmenopausal women, QUS parameters were positively influenced by antiresorptive agents or supplementation with calcium and vitamin D [20, 21]. However, critics have questioned the ability of QUS to detect treatment response because of its lower precision and changes over time with respect to DXA [13].
To date, no data exist in the literature on the effects of teriparatide on QUS parameters. The aims of the present study were twofold: (1) to determine whether teriparatide treatment influences QUS parameters at the calcaneus and phalanxes in postmenopausal women with established osteoporosis, and (2) to compare the changes of QUS with those of BMD by DXA at the axial and appendicular (hand) skeleton.
Subjects and methods
Subjects
This randomized prospective, parallel-group, open-label study was carried out using a cohort of 60 postmenopausal women at least 5 years after natural menopause, age 71.1±6.8 years with established osteoporosis, who were consecutively referred to the Centre of Prevention, Diagnosis and Therapy of Metabolic Bone Diseases at the University of Siena (Italy). All patients were ambulatory and had been under treatment with antiresorptive drugs for at least 12 months. Woman were excluded if they had suffered clinical fractures in the 3 months before the study. Patient recruitment begun in March 2004 and was concluded in June 2004.
Patients were not more than 15% below or 30% above ideal body weight, and they had spine anatomy suitable for obtaining DXA measurements of at least three lumbar vertebrae.
We excluded patients with vitamin D deficiency (defined as serum 25-hydroxy vitamin D concentration <15 ng/ml) or with metabolic disorders known to affect mineral metabolism. Other exclusion criteria were history of alcohol abuse (>300 g/week); habitual smoking (>15 cigarettes/day); cancer (except nonmelanoma skin cancer); stroke or transient ischemic attack; nephrolithiasis or urolithiasis during the previous 2 years; significantly impaired renal or hepatic function; diabetes; or intake of drugs known to interfere with calcium metabolism, such as anticonvulsants, corticosteroids, heparin, anabolic steroids, and gonadic hormones.
The preceding antiresorptive treatment was represented by raloxifene (60 mg/day orally) in 14 patients, tibolone in five, salmon calcitonin (100 IU intramuscularly every other day) in six, clodronate (100 mg/week intramuscularly) in 15, risedronate (35 mg/week) in eight, and alendronate (70 mg/week) in 12. On recruitment, most patients were already receiving calcium supplementation (generally 500 mg/day).
The study was approved by the local ethics committee, and all participants gave informed consent. The protocol consisted of two phases: a run-in phase of a minimum of 1 month and a maximum of 2 months, and a treatment phase of 12 months. During the run-in phase we ensured that all patients received daily supplementation of calcium (1,000 mg) and vitamin D (400 IU); study participants continued with these calcium and vitamin D supplements throughout the entire study period. At the end of the run-in phase, participants were randomly assigned to either receive 20 μg teriparatide (Forsteo; Eli Lilly) subcutaneous injection once daily (n=30) or continue the previous antiresorptive treatment (n=30).
Fifty-five patients (27 in the teriparatide group and 28 in the control group) completed the 12-month study period, and five patients withdrew from the study for problems unrelated to the study drugs. Only the results from the women with complete 12-month follow-up are analyzed and reported here.
Methods
QUS measurements were carried out at the calcaneus using the Achilles Plus (GE, Lunar USA) and at phalanxes using the Bone Profiler (IGEA Italy) at the end of the run-in phase (baseline) and after 2, 4, 6, 8, 10, and 12 months of treatment.
The Achilles Plus measures speed of sound (SOS), broadband ultrasound attenuation (BUA), and a clinical index called stiffness. Stiffness is calculated automatically by the machine according to the following formula: stiffness =(0.67×BUA+0.28×SOS)−420. The Bone Profiler measures the amplitude-dependent SOS (AD-SoS; m/s) and some parameters of the graphic trace of the QUS signal [19, 22]. Among these, we considered bone transmission time (BTT; μs), which is the difference between the time when the first peak of the signal received attains its maximum and the time that would be measured if no bone, but only soft tissue, were present between the transducers, and fast wave amplitude (FWA; mV), which is the maximum amplitude of the fastest peak of the received ultrasound signal. AD-SoS depends on the signal amplitude because it is calculated by considering the time when the electrical signal, generated by the ultrasound mechanical wave at the receiving probe, reaches an amplitude of 2 mV [22]. In our institution the precision of the QUS parameters evaluated in postmenopausal osteoporotic women was 1.7% for stiffness, 0.8% for AD-SoS, 0.6% for BTT, and 6% for FWA. In addition, the standardized coefficient of variation (sCV) was calculated for each QUS parameter according to the following formula: sCV = CV%/range/mean, where range was the difference between the 5th and the 95th percentiles of the population. The sCV were 2.9% for stiffness, 4.4% for AD-SoS, 11.9% for FWA, and 1.1% for BTT.
In all subjects at baseline and at months 6 and 12, we used DXA (Prodigy, GE, Lunar) to measure BMD at the lumbar spine (BMD-LS), at femoral subregions (femoral neck: BMD-FN; total hip: BMD-T; trochanter: BMD-TR), at total body (BMD-TB), and at the right hand (BMD-H). Figure 1 shows the analysis of the total hand performed by using the manual mode as described by Brownbill and Ilich [23]. BMD at the right hand was also measured at months 2, 4, 8, and 10. In our institution the in vivo precision of BMD-H, as obtained by measuring 50 postmenopausal women twice with repositioning, was 1.1%. During the study period no devices were exchanged or modified in their hardware or software components. All measurements were performed by the same operator, who was blinded to the group characteristics.
Statistical analysis
Clinical data and initial values of the measured variables in the study groups were compared using Student’s t-test for unpaired data. For BMD and QUS parameters, the absolute changes over time for each woman were expressed as a percentage of the baseline values. Two-tailed paired t-tests and Wilcoxon matched-pairs signed-ranks tests were used, when appropriate, to compare the changes at each time point with the baseline values. Two-tailed Student’s t-test and Mann-Whitney U-test were used to compare the difference between patient groups. A p-value <0.05 was considered statistically significant.
Results
The baseline characteristics of the 55 patients who completed the study period are shown in Table 1. There were no significant differences between the two groups in baseline characteristics. The mean BMD values at the lumbar spine and femoral neck were −3.5 and −2.6 standard deviations below the respective mean values for young women. In the teriparatide patients, the previous antiresorptive treatment was represented by alendronate in six, risedronate in three, raloxifene in six, tibolone in two, salmon calcitonin in three, and clodronate in seven.
The time course of changes in BMD at the axial skeleton and whole body over the 1-year study period is shown in Table 2. In teriparatide-treated patients, BMD-LS significantly increased by 5.6% and by 7.1% after 6 and 12 months, respectively, whereas in the control group it increased by 1.2% and 1.5%, respectively. The difference between the two groups was significant (p<0.05) after both 6 and 12 months. At month 6 in the teriparatide patients, both BMD-FN and BMD-T were reduced with respect to baseline (−1.8% and −2.1%, respectively). During the following 6 months, BMD-T tended to increase but remained lower with respect to baseline (−0.8%). In contrast, in the last 6 months of teriparatide treatment, BMD-FN markedly increased and at month 12 was significantly higher with respect to baseline (2.6%, p<0.05). In the control group, neither BMD-FN nor BMD-T showed any significant change during the study period. The changes in BMD-TB were −0.4% and −0.6%, respectively, after 6 and 12 months of teriparatide treatment. Corresponding changes in the patients on antiresorptive treatment were 0.2% and 0.3%, respectively. None of the changes was statistically significant.
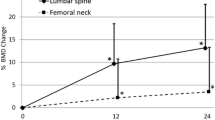
The changes in BMD-H are shown in Fig. 2. The teriparatide group already showed a significant decrease in BMD-H after 4 months of therapy. At month 6, BMD-H was 3.6% lower with respect to baseline; BMD-H tended to increase thereafter, but at month 12 it remained reduced with respect to baseline (−2.7%). The differences between the two groups were significant at both months 6 and 12 (Fig. 2).
Figure 3 shows the changes in QUS parameters at the calcaneus and at phalanxes. Stiffness index showed no significant change in either of the two groups (Fig. 3a). Stiffness decreased during the first 6 months of teriparatide treatment (−1.5% at month 6), and thereafter it tended to return to baseline values (0.1% at month 12). In patients continuing the treatment with antiresorptive drugs, stiffness showed a progressive but not significant increase (Fig. 3a). In the teriparatide group, SOS remained substantially unchanged throughout the study period, whereas BUA decreased in the first 6 months and thereafter tended to increase (data not shown). None of the changes in QUS parameters at the calcaneus was significant.
Mean (±SE) percent changes in stiffness at the calcaneus (stiffness; a), amplitude-dependent speed of sound at phalanxes (AD-SoS; b), bone transmission time (BTT; c), and first wave amplitude (FWA; d) in osteoporotic women treated for 1 year with teriparatide or antiresorptive drugs (controls) (*p<0.05, **p<0.01, ***p<0.001 vs baseline; #p<0.05, ##p<0.01 between groups)
In the teriparatide group, AD-SoS slightly decreased at month 2 (−0.9%, NS) and at month 4 (−1.3%, p<0.05). Thereafter it tended to increase, and at the end of the study period it was higher with respect to baseline (+0.7%). No notable changes in AD-SoS were observed in patients continuing antiresorptive treatment (Fig. 3b).
The QUS parameters derived from the graphic trace analysis at phalanxes (BTT and FWA) showed a divergent pattern in patients treated with teriparatide (Fig. 3c and d). In fact, BTT already showed a significant decrease (−12.2%, p<0.01) after 2 months of teriparatide treatment and remained significantly reduced for the remainder of the study period (Fig. 3c). FWA showed an opposite pattern; in fact, it increased by 9.1% at month 2 even though the increase in FWA values reached statistical significance only at month 6 (13%, p<0.01), thereafter remaining elevated until the end of the study (Fig. 3d). In patients treated with antiresorptive drugs, FWA remained unchanged, and BTT showed a slight increase (Fig. 3c and d). Combining BTT and FWA in a ratio, we found that teriparatide was able to significantly (p<0.01) increase the FWA/BTT ratio by 26.6% and 32.9% after 6 and 12 months, respectively. In contrast, the FWA/BTT ratio remained substantially unchanged in women treated with antiresorptive drugs.
Discussion
Our results clearly show that 1-year treatment with teriparatide in postmenopausal women who were previously treated with antiresorptive drugs induced spine BMD increases similar to those observed in the Fracture Prevention Trial, when teriparatide was given to naïve patients [5], and to those observed in the study by Ettinger et al. [12] in patients previously treated with raloxifene. In the same study, Ettinger et al. [12] reported that patients previously treated with alendronate showed an increase in BMD-LS of about half that observed in naïve patients. There is growing evidence from some [12, 24], but not all [25], studies that prior therapy with alendronate may blunt the effectiveness of teriparatide by reducing the expected increments in both turnover and density of bone. No clinical information currently exists regarding interactions between other bisphosphonates or calcitonin and teriparatide [26]. Raloxifene and estrogen, instead, do not seem to blunt the anabolic effect of PTH [12, 26]. Unfortunately, in our study the low number of patients does not allow any separate analysis on the basis of the prior treatments.
In our study, teriparatide treatment was associated with an early decrease in BMD-FN with a subsequent tendency to reverse this trend. Similar biphasic patterns of BMD at the femur were reported in osteoporotic women receiving hPTH (1–84) [27, 28] and in those receiving hPTH (1–34) who had been previously treated with alendronate [12]. The disparate effects of PTH on trabecular and cortical bone may explain the fact that BMD-TB is reported as slightly increased in some studies [5] and slightly decreased in others [27, 28].
To our knowledge, this is the first study to evaluate the effect of teriparatide on BMD at the hand, an appendicular site characterized by a high percentage of cortical bone [23], showing that BMD-H was markedly reduced in the teriparatide-treated group. Early reduction in BMD as measured by DXA at skeletal sites where cortical bone prevails was reported either in patients treated with teriparatide [3–5] or intact PTH [27, 28] and is probably due to an enhanced intracortical remodeling leading to the “trabecularization” of the endocortical envelope. However, it has been reported that teriparatide could offset the expansion of the inner diameter of tubular bones by an increase in external diameter due to new periosteal bone apposition [6, 26]. The possible negative effect of teriparatide on areal BMD may be increased in patients previously on long-term bisphosphonate treatment. In fact, the latter may reduce the ability of PTH to stimulate new bone formation [24]. Because BMD measurement by DXA presents several shortcomings in evaluating the effects of PTH on bone, there is a pressing need to explore the utility of other complementary techniques (e.g., QCT, magnetic resonance imaging, or QUS) [26].
Our study, even though preliminary and carried out on a small sample of patients, suggests the possibility of using QUS parameters, namely BTT and FWA, to monitor the effects of teriparatide on bone. These derive from the analysis of ultrasound received signals, and each reflects somewhat different aspects of skeletal status [29]. BTT is independent of the thickness of the soft tissue, depends on the amount of bone inside the phalanxes, and is strongly correlated to pure SOS, so the results for SOS should also be valid for BTT [29, 30]. It has been reported that BTT can be considered sensitive to cortical bone density, cortical thinning, and porosity [30]. In our patients, the reduction in BTT may be a combination of several effects induced by PTH, which include increased endocortical remodeling, expansion of the inner diameter, and, probably, the “trabecularization” of the endocortical envelope. In a previous study we found that BTT was markedly more reduced in patients with primary hyperparathyroidism than in osteoporotic women, with respect to controls [19].
On the other hand, the significant increase in BTT observed by Ingle et al. [31] in osteoporotic women, in the course of a 2-year alendronate treatment, could be explained by the increased mineralization and reduced endosteal resorption due to bisphosphonate treatment.
The significance of the increase in FWA is less evident. Barkmann et al. [29] showed that in healthy subjects, FWA was related to the medullary canal cross-sectional area but not to the cortical area. In a previous study we found that FWA was significantly reduced in osteoporotic patients but not in patients with primary hyperparathyroidism, and we suggested that FWA was probably related to trabecular connectivity, which is conserved in hyperparathyroidism [19]. Moreover, it has been reported that FWA is associated with the structure of the trabecular bone [32]. The FWA increase in teriparatide-treated patients may reflect the deposition of new bone with a consequent improvement in bone connectivity [33].
Therefore, because BTT and FWA are differently influenced by teriparatide, the FWA/BTT ratio could better express the global effect of teriparatide on bone. The pattern of AD-SoS, the QUS variable routinely measured in clinical practice, may be explained by the fact that it is affected by both the relative cortical area and the signal amplitude [29]. Moreover, it has been reported that AD-SoS depends less on cortical area than BTT [29, 30]. AD-SoS showed a decrease during the first 6 months of teriparatide therapy, and thereafter it tended to increase and could have been expected to further increase if the observation period had been prolonged parallel with the mineralization of the newly formed bone. The reproducibility represents one of the major critical points for the use of QUS in clinical practice. The precision data for QUS parameters at phalanxes are quite different in the various studies that evaluated different populations. Namely, the CV of BTT is better in our study with respect to that reported by other authors [31]. However, it is important to consider that the precision of QUS parameters could lessen when using QUS devices in a real practice setting outside of a dedicated laboratory. Despite substantial increases in spine BMD in the teriparatide group, parallel increases in calcaneal QUS measurements did not occur, even though both sites are predominantly trabecular bone and weight bearing. The clinical significance of these findings is unknown. However, the mild and transient decrease in the stiffness index at the calcaneus could be explained by an increased porosity of the cortical envelope.
Clearly, our study presents some limitations: Our sample size was relatively small, the observation period was limited to 1 year, and the reproducibility of some QUS parameters, namely FWA, is not entirely satisfactory. Moreover, the patients included in the study were not naïve. However, this latter could also be considered a point of strength because our study population is representative of normal patients who are generally treated with antiresorptive drugs before receiving teriparatide.
In conclusion, our study shows that in women with established osteoporosis who had previously been treated with various antiresorptive drugs, a 1-year teriparatide treatment determined the expected increase in BMD at the axial skeleton and a significant and prolonged decrease in BMD at the hand. Moreover, teriparatide determined important changes in BTT and FWA, two parameters obtained from the analysis of ultrasonographic trace at phalanxes, which could be considered in monitoring for the effect of teriparatide on bone. Further studies carried out on a larger population would be necessary to define the clinical role of QUS parameters in patients treated with teriparatide.
References
Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C (2002) Meta-analyses of therapies for postmenopausal osteoporosis. IX. Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev 23:570–578
Finkelstein JS, Klibanski A, Schaefer EH, Hornstein MD, Schiff I, Neer RM (1994) Parathyroid hormone for prevention of bone loss induced by estrogen deficiency. N Engl J Med 331:1618–1623
Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP (2000) Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab 85:3069–3076
Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perz A, Kaufman JM, Clancy AD, Gaich GA (2003) The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18:9–17
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Zanchetta JR, Bogado CE, Ferretti JL, Wang O, Wilson MG, Sato M, Gaich GA, Dalsky GP, Myers SL (2003) Effects of teriparatide [recombinant human parathyroid hormone (134)] on cortical bone in postmenopausal women with osteoporosis. J Bone Miner Res 18:539–543
Rehman Q, Lang TF, Arnaud CD, Modin GW, Lane NE (2003) Daily treatment with parathyroid hormone is associated with an increase in vertebral cross-sectional area in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int 14:77–81
Burr DB, Hirano T, Turner CH, Hotchkiss C, Brommage R, Hock JM (2001) Intermittently administered human parathyroid hormone(134) treatment increases intracortical bone turnover and porosity without reducing bone strength in the humerus of ovariectomized cynomolgus monkeys. J Bone Miner Res 16:157–165
Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetic K, Muller R, Bilezikian J, Lindsay R (2001) Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res 16:1846–1853
Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF (2003) Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res 18:1932–1941
Adami S, Viapiana G, Gatti D (2004) Bone anabolic agents: the unanswered queries. Basic Clin Pharmacol Toxicol 94:257–259
Ettinger B, San Martin J, Crans G, Pavo I (2004) Differential effects of teriparatide on bMD after treatment with raloxifene or alendronate. J Bone Miner Res 19:745–751
Gluer CC, for the International Quantitative Ultrasound Consensus Group (1997) Quantitative ultrasound techniques for the assessment of osteoporosis: expert agreement on current status. J Bone Miner Res 12:1280–1288
Njeh CF, Fuerst T, Diessel E, Genant HK (2001) Is quantitative ultrasound dependent on bone structure? A reflection. Osteoporos Int 12:1–15
Hans D, Dargent-Molina P, Schott AM, Sebert JL, Cormier C, Kotzki PO, Delmas PD, Pouilles JM, Breart G, Meunier PJ, for the EPIDOS prospective study group (1996) Ultrasonographic heel measurements to predict hip fracture in elderly women: the EPIDOS prospective study. Lancet 348:511–514
Bauer DC, Gluer CC, Cauley JA, Vogt TM, Ensrud KE, Genant HK, Black DM, for the study of osteoporotic fractures research group (1997) Broadband ultrasound attenuation predicts fractures strongly and independently of densitometry in older women: a prospective study. Arch Intern Med 157:629–634
Mulleman D, Legroux-Gerot I, Duquesnoy B, Marchandise X, Delcambre B, Cortet B (2002) Quantitative ultrasound of bone in male osteoporosis. Osteoporos Int 13:388–393
Gonnelli S, Cepollaro C, Gennari L, Montagnani A, Caffarelli C, Merlotti D, Rossi S, Cadirni A, Nuti R (2005) Quantitative ultrasound and dual-energy X-ray absorptiometry in the prediction of fragility fracture in men. Osteoporos Int 16:963–968
Montagnani A, Gonnelli S, Cepollaro C, Bruni D, Franci MB, Lucani B, Gennari C (2002) Graphic trace analysis of quantitative ultrasound at phalanxes seems to improve the diagnosis of primary hyperparathyroidism among patients with low bone mass. Osteoporos Int 13:222–227
Krieg MA, Jacquet AF, Bremgartner M, Cuttelod S, Thieband D, Burckardt P (1999) Effect of supplementation with vitamin D3 and calcium on quantitative ultrasound of bone in elderly institutionalized women: a longitudinal study. Osteoporosis Int 9:483–488
Gonnelli S, Cepollaro C, Montagnani A, Martini S, Gennari L, Mangeri M, Gennari C (2002) Heel ultrasonography in monitoring alendronate therapy: a four-year longitudinal study. Osteoporos Int 13:415–421
Wuster C, Albanese C, De Aloysio D, Duboeuf F, Gambacciani M, Gonnelli S, Gluer CC, Hans D, Joly J, Reginster JY, De Terlizzi F, Cadossi R (2000) Phalangeal osteosonogrammetry study: age-related changes, diagnostic sensitivity, and discrimination power. The Phalangeal Osteosonogrammetry Study Group. J Bone Miner Res 15:1603–1614
Brownbill RA, Ilich JZ (2002) Validation of the use of the hand for estimating bone mineral density in other skeletal sites by DXA in healthy and osteoarthritic women. J Clin Densitom 5:273–282
Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM (2003) The effects of parathyroid hormone, alendronate, or both in men whit osteoporosis. N Engl J Med 349:1216–1226
Cosman F, Nieves JW, Luckey MM, Zion M, Woelfert L, Lindsay R (2003) Daily versus cyclic PTH combined with alendronate versus alendronate alone for treatment of osteoporosis. J Bone Miner Res 18(Suppl 2):S32
Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, Mc Clung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK (2005) Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev 26:688–703
Rittmaster RS, Bolognese M, Ettinger MP, Hanley DA, Hodsman AB, Kendler DL, Rosen CJ (2000) Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab 85:2129–2134
Hodsman AB, Hanley DA, Ettinger MP, Bolognese MA, Fox J, Metcalfe AJ, Lindsay R (2003) Efficacy and safety of human parathyroid hormone (1–84) in increasing bone mineral density in postmenopausal osteoporosis. J Clin Endocrinol Metab 88:5212–5220
Barkmann R, Lusse S, Stampa B, Sakata S, Heller M, Gluer CC (2000) Assessment of the geometry of human finger phalanges using quantitative ultrasound in vivo. Osteoporos Int 11:745–755
Sakata S, Barkmann R, Lochmuller EM, Heller M, Gluer CC (2004) Assessing bone status beyond BMD: evaluation of bone geometry and porosity by quantitative ultrasound of human finger phalanges. J Bone Miner Res 19:924–930
Ingle BM, Machado AB, Pereda CA, Eastell R (2005) Monitoring alendronate and estardiol therapy with quantitative ultrasound (QUS) and bone mineral density. J Clin Densitom 8:278–286
Hosokawa A, Otani T (1997) Ultrasonic wave propagation in bovine cancellous bone. J Acoust Soc Am 101:558–562
Misof BM, Roschger P, Cosman F, Kurland ES, Tesch W, Messmer P, Dempster DW, Nieves J, Shane E, Fratzl P, Klaushofer K, Bilezikian J, Lindsay R (2003) Effects of intermittent parathyroid hormone administration on bone mineralization density in iliac crest biopsies from patients with osteoporosis: a paired study before and after treatment. J Clin Endocrinol Metab 88:1150–1156
Acknowledgement
We would like to thank Dr. Francesca De Terlizzi (IGEA Biophysics Laboratory, Carpi, Italy) for her valuable technical assistance in the revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonnelli, S., Martini, G., Caffarelli, C. et al. Teriparatide’s effects on quantitative ultrasound parameters and bone density in women with established osteoporosis. Osteoporos Int 17, 1524–1531 (2006). https://doi.org/10.1007/s00198-006-0157-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-006-0157-3