Abstract
Introduction
Despite the efficacy of bisphosphonates to reduce fractures in high risk populations, bisphosphonate adherence among chronic glucocorticoid users has received limited attention. Moreover, perceived differences in GI tolerability may lead physicians to preferentially prescribe particular bisphosphonates.
Methods
Among chronic glucocorticoid users (>60 days of therapy) enrolled in managed care, we identified individuals initiating therapy with alendronate or risedronate during 2001–2004. Multivariable logistic regression and proportional hazards models were used to examine factors associated with channeling patients to risedronate (versus alendronate) and with discontinuation (>3-month gap without refill). The Medication Possession Ratio (MPR) was calculated as the filled days of medication divided by the interval of time between prescriptions.
Results
Of 1,158 glucocorticoid users initiating bisphosphonate therapy, demographic characteristics of alendronate users (n=754) and risedronate users (n=404) were similar for age (mean 53 years) and gender (approximately 80% female). Past history of a GI symptom or event was associated with risedronate receipt (OR=2.24, 95% CI 1.15–4.35). After multivariable adjustment, rates of discontinuation (mean time to discontinuation approximately 18 months) and adherence (mean MPR=73%) were similar between users of the two bisphosphonates. Younger age, greater medical comorbidity, and lack of BMD testing were significantly associated with discontinuation.
Conclusions
Overall persistence rates were suboptimal for bisphosphonate use among chronic glucocorticoids users and did not differ significantly by drug. Newer strategies to promote long-term adherence are needed to improve osteoporosis therapeutic effectiveness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the efficacy of the oral bisphosphonates alendronate and risedronate in reducing fracture risk [1, 2], associated gastrointestinal (GI) side effects are relatively common and may lead to discontinuation [3]. Among other factors, perceived variations in GI tolerability between agents may lead physicians to select one bisphosphonate over another (prescription channeling). Premature discontinuation (low persistence) and not taking medications according to the prescribed dosing regimen (low adherence) with bisphosphonate therapy has been associated with increased fracture risk in postmenopausal women [4]. The availability of once-weekly bisphosphonate dosing [5] may improve adherence and thus the effectiveness of these medications, although there are limited data supporting this premise. However, the relationship between physician selection of particular bisphosphonates, their perceived GI tolerability, and subsequent patient persistence and adherence have been minimally studied in populations at high risk for osteoporotic fractures such as individuals receiving chronic glucocorticoid therapy.
Among chronic glucocorticoid users enrolled in a U.S. managed care plan, we hypothesized that persistence and adherence with alendronate and risedronate therapy would be suboptimal and would differ between the two drugs. Moreover, based on both endoscopy [6] and observational study data [7, 8] suggesting that risedronate may have better GI tolerability than alendronate, we hypothesized differences between the perceived tolerability of these agents. Depending on physicians’ perception of the bisphosphonate GI adverse event data, higher risk patients might be channeled to risedronate, but only limited information exists about whether this actually occurs in clinical practice. Channeling might have a significant impact on adverse event and discontinuation rates if patients at greater risk for GI events and discontinuation preferentially receive risedronate. We therefore examined whether individuals that were at higher risk for upper gastrointestinal symptoms or events based on their past medical histories might be preferentially channeled to risedronate and whether channeling affected discontinuation.
Methods
Data sources
Chronic glucocorticoid users were identified in 2001–2003 from a national managed care organization (MCO) population covering approximately 13 million people across the U.S. The data source consisted of linked enrollment, outpatient encounter, pharmacy, and procedural billing databases for enrollees in the MCO’s health maintenance organization (HMO), point of service (POS), and preferred provider organization (PPO) health plans. Diseases, medications, and procedures were coded using International Classification of Diseases 9th revision codes (ICD-9), National Drug Codes (NDC), and Common Procedural Terminology (CPT-4) codes, respectively. All individuals in the cohort had insurance coverage for medications although individuals may have been required to pay at least some portion of the medication cost (a co-payment).
Identification of chronic glucocorticoid users
Individuals were characterized as chronic glucocorticoid users if they were 18+ years of age and had received 60 or more days of outpatient oral glucocorticoids from 1 July 2001 to 30 June 2003 (the glucocorticoid eligibility period). All members were continuously enrolled in the health plan for at least 6 months prior to and following their first qualifying glucocorticoid prescription and throughout the eligibility period. We identified the physician prescribing glucocorticoids based on Drug Enforcement Agency numbers listed on filled glucocorticoid prescriptions. Because we were interested in the bisphosphonate prescribing habits of physicians of various specialties, we oversampled specialists that commonly prescribe glucocorticoids. A total of 6,282 health plan members met the inclusion criteria for chronic glucocorticoid use and were selected for further study.
Identification of new bisphosphonate users
Among the 6,282 chronic glucocorticoid users, we defined new bisphosphonate users as individuals that filled their first oral bisphosphonate prescription between 1 July 2001 to 30 June 2004. We required that eligible individuals be new bisphosphonate users with no filled prescriptions in the 180 days prior to the date of their first bisphosphonate prescription (the index date). Individuals receiving oral bisphosphonates at doses used to treat Paget’s disease of bone (i.e., alendronate 40 mg daily, risedronate 30 mg daily) were excluded. Bisphosphonate dosing was characterized as “weekly” if all filled bisphosphonate prescriptions were prescribed once weekly. New bisphosphonate users that subsequently received the “other” bisphosphonate (i.e., “switchers”) were assigned to the cohort of their initial drug exposure only, since individuals intolerant to one bisphosphonate might have a higher risk of intolerance to a second bisphosphonate.
Factors associated with channeling, adherence, and endpoints of interest
To assess factors that might lead physicians to select one bisphosphonate over the other, we examined the pharmacy and administrative data for selected medications and medical conditions prior to first receipt of bisphosphonate therapy. These included medications used to treat GI symptoms and diseases (i.e., H2 blockers, proton pump inhibitors, and misoprostol) and physician visits with pre-specified GI ICD-9 codes (see Appendix 1) [7]. Because nonsteroidal anti-inflammatory drugs (NSAIDs) may pose an additional risk for bisphosphonate-associated GI toxicity, we examined the pharmacy database for NSAID use prior to the initiation of bisphosphonate therapy.
We examined adherence with bisphosphonate therapy and defined adherence by the medication possession ratio (MPR) [9]. The MPR was calculated as the number of bisphosphonate fill days dispensed divided by the interval between the first and last bisphosphonate prescriptions. Adherence was capped at 100% to prevent bias from some patients who “stockpile” medication. Because at least two filled prescriptions were required in order to calculate this metric, individuals that filled only 1 prescription (n=235) were not represented in this endpoint but were included in all other results. The MPR represents adherence between filled prescriptions but does not consider the total number of filled prescriptions, so we separately examined bisphosphonate discontinuation. We defined discontinuation (i.e. nonpersistence) as more than a 3-month gap after the number of fill days of the last prescription. Because very lengthy final prescriptions might limit our ability to assess discontinuation, we excluded individuals that were dispensed more than 3 months of medication on their last fill date (n=4).
Statistical analyses
Categorical variables were compared using the chi-square test of independence, and continuous variables were compared using a two-tailed t-test. Multivariable logistic regression was used to identify factors associated with receipt of risedronate rather than alendronate (channeling). Variables of high clinical interest (e.g., physician specialty, prior gastrointestinal event) were forced into the regression models. For all other variables, a univariate P value <0.25 was required for entry into the model and a P value <0.05 was required to remain in the model. Model building was conducted according to Hosmer and Lemeshow [10]. Goodness of fit and model calibration for logistic regression were assessed using the Hosmer-Lemeshow goodness of fit and c statistics, respectively [10, 31]. Time to discontinuation was examined using Kaplan-Meier curves and compared using the log-rank test. Model building using proportional-hazards survival-analysis methods [32] was performed similarly to that described for the logistic regression models. All analyses were performed using SAS (SAS Institute, Cary, NC).
Results
Among the 6,282 managed care enrollees who were prescribed at least 60 days of glucocorticoids, 1,158 were new bisphosphonate users. Their characteristics prior to first receipt of alendronate or risedronate and during the study period are shown in Table 1. Individuals in each group were relatively young (mean age 53 years), predominantly women, and had a mean of 39 months of observation time in the study, approximately 1/2 of which was following initiation of bisphosphonate therapy. The burden of concomitant illness was high as assessed by the summed comorbidity count and the number of unique medications. Almost two-thirds of the study cohort received bone-mass measurement at some time during the study period, and approximately 10% had one or more administrative claims for a fracture. Risedronate users were more likely to have seen a physician for a GI symptom or event and to have received a gastrointestinal medication prior to beginning bisphosphonate therapy. A greater proportion of alendronate users received once-weekly bisphosphonate rather than once-daily.
Table 2 shows multivariable-adjusted factors independently associated with receipt of risedronate (i.e., channeling). Previous history of a GI symptom or event increased the odds of risedronate receipt by more than two-fold. A longer duration of time in the health plan was also associated with risedronate receipt. Compared to patients of internists and rheumatologists, patients receiving glucocorticoids from physicians of other specialties were somewhat less likely to receive risedronate.
Table 3 describes rates of GI events, GI medication use, and adherence (MPR) after beginning bisphosphonate therapy. Almost one-fourth of patients in each group experienced a GI symptom or event after initiating therapy, and adherence was similar between alendronate and risedronate users. Among the individuals who discontinued, 14% subsequently filled prescriptions for the other oral bisphosphonate during the study period (data not shown). Mean adherence was slightly greater among individuals prescribed weekly therapy compared to those prescribed daily therapy (74 vs. 68%, P<0.05; data not shown).
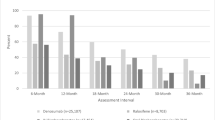
The Kaplan-Meier curves in Fig. 1 demonstrate that there were no significant differences in the unadjusted time to discontinuation (i.e., non-persistence) between alendronate and risedronate users. Approximately half of the cohort had discontinued bisphosphonates by 18 months. Table 4 describes factors associated with time to discontinuation. Concordant with the results from the unadjusted Kaplan-Meier curves, there were no significant differences in the time to discontinuation associated with the particular bisphosphonate prescribed after multivariable adjustment. Those persons receiving glucocorticoids for longer periods of time were less likely to discontinue, as were older individuals and those who received bone mineral density testing. Individuals with a higher burden of comorbidity were more likely to discontinue. A GI symptom or event prior to first bisphosphonate use had no significant association with subsequent discontinuation. Moreover, we did not find that individuals with a GI symptom or event following initiation of bisphosphonate therapy were significantly more likely to discontinue (data not shown).
Discussion
Glucocorticoid use is an independent risk factor for fracture irrespective of its deleterious effects on BMD [11]. Thus, chronic glucocorticoid users are an important target population for osteoporosis prevention efforts. Among chronic glucocorticoid users who initiated oral bisphosphonate therapy, we found that individuals with a prior history of gastrointestinal medication use or illness were more than twice as likely to receive risedronate compared to alendronate, suggesting channeling. We found that alendronate and risedronate users had similar adherence (MPR) during the time that they were on therapy, and after multivariable adjustment accounting for channeling bias and other factors, there was no difference in discontinuation between alendronate and risedronate users.
Most studies of osteoporosis medication use in chronic glucocorticoid users have focused on determinants of receipt of any osteoporosis medication rather than the particular medication chosen [12–14]. Thus, there is limited literature with which to compare our results showing that individuals at higher risk for gastrointestinal symptoms appeared to be channeled to risedronate. Data from a head-to-head randomized controlled trial (RCT) of postmenopausal women suggest that there is equal tolerability between agents [15]. In contrast, endoscopy data and short term observational studies of managed care enrollees initiating bisphosphonate therapy showed that risedronate use is associated with fewer ulcers, GI events and GI-related medical resources than alendronate use [6–8]. Our results are consistent with the idea that physician’s perceptions about the comparative GI tolerability of the two bisphosphonates may significantly affect prescribing choices. However, our data did not show that a prior GI event was a strong risk factor for subsequent bisphosphonate discontinuation, and therefore the effect of this channeling on our results in Table 4 was small. We also found that longer time enrolled in the health plan was also associated with risedronate receipt. One possible explanation for this finding is that risedronate was FDA-approved after alendronate, and diffusion of newer therapies into practice increases over time.
Compared to channeling, discontinuation rates for bisphosphonates have received somewhat more attention, albeit in populations other than chronic glucocorticoid users. Irrespective of which bisphosphonate was chosen, adherence and persistence with bisphosphonate therapy in our cohort were better than in most prior studies of postmenopausal women initiating treatment [16–18]. In earlier studies, mean MPR was estimated at about 60%, and discontinuation rates at 1 year ranged from approximately 60–75%. Similar to those studies, we found small differences in adherence between weekly and daily bisphosphonate users, although dosing frequency was not independently associated with persistence after multivariable adjustment. A few studies have found that alendronate users are more likely to discontinue therapy than users of osteoporosis medications other than risedronate [19, 20]. In contrast, we found no significant differences in the time to discontinuation or in the rate of GI events between alendronate and risedronate users. Indeed, our results showed that patient characteristics, comorbidities, and health service utilization were more strongly associated with persistence than the particular drug taken.
Of high relevance for a patient population with chronic illnesses requiring glucocorticoid use, and in contrast to some findings [17], we showed that an increased burden of medical comorbidity decreased the likelihood that patients would persist with their osteoporosis treatment. Because greater patient comorbidity may reflect increased time pressures on physicians to manage complex patients, strategies to optimize chronic disease management may be useful to increase long-term persistence with osteoporosis therapies [21]. Also in contrast to at least some prior studies, we found that older patients were more likely to persist with therapy. Differences between our results and early studies likely reflect variations among underlying patient populations. Our study included a broader age range of individuals with chronic diseases requiring chronic glucocorticoid therapy (e.g., rheumatoid arthritis), and individuals with these diseases likely have different patterns of medication adherence compared to the general population.
Concordant with data showing that BMD and bone-biomarkers testing improves adherence [22, 23], we found that individuals who received a BMD test were more likely to persist with therapy. BMD testing may motivate patients to remain compliant, may reflect patients’ improved understanding of being at-risk for osteoporosis, or may simply be a surrogate for patients or physicians who are concerned about osteoporosis and more likely to remain persistent with treatment. Of particular methodologic importance is our observation that the mean adherence of 73% overall is very close to an 80% adherence cutpoint used in some osteoporosis studies [16]. Although limited data have shown improved endpoints using this somewhat arbitrary but commonly-accepted definition [4], our results suggest that the 80% threshold is likely to result in substantial misclassification and should be avoided until finer gradations of physiologically relevant thresholds can be determined based on fracture endpoints.
The strengths of our study include a new bisphosphonate user study design of individuals initiating bisphosphonate therapy followed for longer periods of time than other studies that restrict observation time to 1 year. As described above, our unique patient population of chronic glucocorticoid users seen in a real-world setting is complementary to other studies focusing on persistence in cohorts of predominantly older postmenopausal women or those enrolled in RCTs. Despite possible differences between pharmacy data estimates and what patients actually take, we have previously shown that concordance between the pharmacy data and self-reported current bisphosphonate use was excellent, with kappa values of approximately 0.8 [24]. Finally, in contrast to some studies that define discontinuation as a gap of >30 days [17], our more conservative definition of discontinuation (i.e. more than a 3-month gap following the length of the last filled prescription) limits misclassification of discontinuation for patients who may temporarily interrupt therapy but shortly resume.
Despite these strengths, administrative and pharmacy data can provide only limited information on why physicians choose particular therapies or why patients discontinue therapy. Indeed, data from studies of other classes of medications such as NSAIDs suggest that patients’ measurable medical comorbidities have only a limited impact on prescribing decisions and may be significantly outweighed by physician characteristics that are difficult to enumerate [25, 26]. Concordant with this notion, the c statistics and R 2 values for our models suggest that there are other factors that influence both prescribing and discontinuation that we were not able to capture using administrative data alone. Among several potential factors, advantageous purchasing contracts, preferred medication lists specific to individual plan members, or marketing influences may have affected prescribing choices. We did not examine fracture incidence related to gradients of persistence and adherence since our sample size was underpowered for this analysis. Although we analytically adjusted for duration of glucocorticoid use in our multivariable discontinuation model, we recognize that the need for osteoporosis prevention is perhaps lessened if patients permanently discontinue glucocorticoids. However, we believed that constructing a valid definition for glucocorticoid discontinuation was problematic since many of the patients in our cohort received intermittent and repeated glucocorticoid tapers, a pattern of use previously associated with significant bone loss [27]. Our study included only managed-care enrollees in the United States and therefore may have limited generalizability to other populations. Finally, although patients may have received osteoporosis medications other than bisphosphonates, we focused only on this very commonly used drug class because of the strength of the evidence supporting their benefit for glucocorticoid-induced osteoporosis [28].
In conclusion, persistence and adherence with bisphosphonate therapy in a population of patients with chronic diseases requiring glucocorticoid use appears to be better than in cohorts of postmenopausal women with osteoporosis. However, it remains suboptimal and does not differ significantly between specific bisphosphonates. For selected patients that discontinue use because of mild gastrointestinal symptoms, medication re-challenge may be appropriate and successful [29, 30]. A combination strategy integrating more frequent physician contact and monitoring as part of a chronic-disease-management program may be useful to promote adherence. Other strategies that utilize osteoporosis medications with less frequent dosing regimens or other routes of administration may be helpful to improve adherence and to reduce the risk of glucocorticoid-induced osteoporosis and related fractures.
References
Black DM, Cummings SR, Karpf DB et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348:1535–1541
Harris ST, Watts NB, Genant HK et al (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA 282:1344–1352
Tosteson AN, Grove MR, Hammond CS et al (2003) Early discontinuation of treatment for osteoporosis. Am J Med 115(3):209–216
Caro JJ, Ishak KJ, Huybrechts KF, Raggio G, Naujoks C (2004) The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int 15(12):1003–1008
Schnitzer T, Bone H, Crepaldi G et al (2000) Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Alendronate once-weekly study group. Aging 12(1):1–12
Lanza FL, Hunt RH, Thomson AB, Provenza JM, Blank MA (2000) Endoscopic comparison of esophageal and gastroduodenal effects of risedronate and alendronate in postmenopausal women. Gastroenterology 119(3):631–638
Kane S, Borisov N, Brixner D (2004) Pharmacoeconomic evaluation of gastrointestinal tract events during treatment with risedronate or alendronate: a retrospective cohort study. Am J Managed Care 10:S216–S226
Miller R, Bolognese M, Worley K, Solis A, Sheer R (2004) Incidence of gastrointestinal events among bisphosphonate patients in an observational setting. Am Journal of Managed Care. 10:S207–S215
Rizzo JA, Simons WR (1997) Variations in compliance among hypertensive patients by drug class: implications for health care costs. Clin Ther 19(6):1446–1457; Discussion 1424–1425
Hosmer DW, Lemeshow S (2000) Applied logistic regression, 2nd ed. Wiley, New York
Kanis JA, Johansson H, Oden A et al (2004) A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res 19(6):893–899
Curtis JR, Westfall AO, Allison JJ et al (2005) Longitudinal patterns in the prevention of osteoporosis in glucocorticoid-treated patients. Arthritis Rheum 52(8):2485–2494
Ettinger B, Chidambaran P, Pressman A (2001) Prevalence and determinants of osteoporosis drug prescription among patients with high exposure to glucocorticoid drugs. Am J Manag Care 7(6):597–605
Yood RA, Harrold LR, Fish L et al (2001) Prevention of glucocorticoid-induced osteoporosis: experience in a managed care setting. Arch Intern Med 161:1322–1327
Rosen CJ, Hochberg MC, Bonnick SL et al (2005) Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 20(1):141–151
Recker RR, Gallagher R, MacCosbe PE (2005) Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc 80(7):856–861
Cramer JA, Amonkar MM, Hebborn A, Altman R (2005) Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin 21(9):1453–1460
Ettinger M, Gallagher R, Amonkar M, Smith J, MacCosbe PE (2004) Medication persistence is improved with less frequent dosing of bisphosphonates, but remains inadequate. Arthritis Rheum 50(9S):S513–S514
Papaioannou A, Ioannidis G, Adachi J et al (2003) Adherence to bisphosphonates and hormone replacement therapy in a tertiary care setting of patients in the CANDOO database. Osteoporos Int 14:S808–S813
Turbi C, Herrero-Beaumont G, Acebes JC et al (2004) Compliance and satisfaction with raloxifene versus alendronate for the treatment of postmenopausal osteoporosis in clinical practice: an open-label, prospective, nonrandomized, observational study. Clin Ther 26(2):245–256
Osterberg L, Blaschke T (2005) Adherence to medication. N Engl J Med 353(5):487–497
Pickney CS, Arnason JA (2005) Correlation between patient recall of bone densitometry results and subsequent treatment adherence. Osteoporos Int 16(9):1156–1160
Clowes JA, Peel NF, Eastell R (2004) The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab 89(3):1117–1123
Curtis J, Westfall A, Allison J, Freeman A, Kovac S, Saag K (2006) Agreement and validity of pharmacy data versus self-report for use of osteoporosis medications among chronic glucocorticoid users. Pharmacoepidemiol Drug Safety (in press)
Schneeweiss S, Glynn RJ, Avorn J, Solomon DH (2005) A Medicare database review found that physician preferences increasingly outweighed patient characteristics as determinants of first-time prescriptions for COX-2 inhibitors. J Clin Epidemiol 58(1):98–102
Solomon DH, Schneeweiss S, Glynn RJ, Levin R, Avorn J (2003) Determinants of selective cyclooxygenase-2 inhibitor prescribing: are patient or physician characteristics more important? Am J Med 115(9):715–720
Haugeberg G, Griffiths B, Sokoll KB, Emery P (2004) Bone loss in patients treated with pulses of methylprednisolone is not negligible: a short term prospective observational study. Ann Rheum Dis 63(8):940–944
American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis (2001) Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheum 44:1496–1503
Hamilton B, McCoy K, Taggart H (2003) Tolerability and compliance with risedronate in clinical practice. Osteoporos Int 14(3):259–262
Miller PD, Woodson G, Licata AA et al (2000) Rechallenge of patients who had discontinued alendronate therapy because of upper gastrointestinal symptoms. Clin Ther 22(12):1433–1442
Lemeshow S, Hosmer JDW (1982) A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol 115:92–106
Hosmer JDW, Lemeshow S (1999) Applied survival analysis: Regression modeling of time to event data. Wiley Series in Probability and Statistics: John Wiley and Sons
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grant number HS10389 from the Agency for Healthcare Research and Quality, P60 AR48095 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, K24 AR052361 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and T32 AR47512-03 from the National Institutes of Health.
Appendix 1 [7]
Rights and permissions
About this article
Cite this article
Curtis, J.R., Westfall, A.O., Allison, J.J. et al. Channeling and adherence with alendronate and risedronate among chronic glucocorticoid users. Osteoporos Int 17, 1268–1274 (2006). https://doi.org/10.1007/s00198-006-0136-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-006-0136-8