Abstract
Introduction
This study evaluated the radiological changes at the bone–cement interface of calcium phosphate cement (CPC) and polymethylmethacrylate (PMMA) 12 months after kyphoplasty. In a pilot experiment, we additionally performed a histomorphometric analysis in osteopenic foxhounds to analyze the process of osseous integration of CPC and PMMA.
Methods
Twenty postmenopausal female patients with 46 vertebral compression fractures (VCF) were treated by kyphoplasty, utilizing CPC (N=28) or PMMA (N=18) for intravertebral stabilization. After a 12-month follow-up, we measured the density changes of border voxels at the bone–cement interface by computed tomography (CT) using dedicated software algorithms. We defined the border-voxel density (BVD) as a parameter of cement resorption at the interface. We also investigated the bone–implant interface in three osteopenic foxhounds by histomorphometry 3, 6, and 12 months after cement implantation.
Results
Twelve months after kyphoplasty, only CPC showed a significant decrease of the BVD compared to PMMA (p<0.01), indicating a slow progress of resorption at the interface. Histomorphometry of the dog vertebrae showed near total bone coverage of CPC implants, whereas the PMMA surface exhibited only 30% direct bone contact (p<0.01). We also observed a time-dependent increase in the number of discernable osteons close to the interface of CPC, but no bone tissue within PMMA (p<0.01).
Conclusions
The decrease of the BVD 12 months after kyphoplasty may indicate osseous integration of CPC by: (1) the ingrowth of bone tissue and (2) osteonal penetration close to the interface.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The treatment of painful vertebral compression fractures (VCF) by kyphoplasty or vertebroplasty has been described for patients with primary osteoporosis [1–4]. In addition to conventional medical treatment of osteoporosis, kyphoplasty allows an immediate and persisting pain relief and, thus, improves mobility in patients suffering from painful VCF [5, 6].
Polymethylmethacrylate (PMMA) is most commonly used in vertebroplasty or kyphoplasty for the stabilization of painful VCF [7–9]. Resorbable calcium phosphate cements (CPC) have been developed as an alternative to allow osseous substitution by autologous bone tissue [10–12]. Recent studies suggest comparable biomechanical properties of both cement types [13–16]. However, the rate of resorption of various CPC materials differs considerably, depending on the chemical composition of the used CPC [17].
It is not known whether CPC is completely substituted by autologous bone tissue after several years. Clinically, it appears advantageous to apply a cement material that is steadily replaced by bone instead of implanting non-resorbable plastic material like PMMA, particularly in younger patients.
In a previous clinical study, we measured the volume changes of resorbable CPC in a 12-month follow-up using computer tomography (CT) volumetry [18]. The measurements were compared with non-resorbable PMMA as a control. The mean volume reduction of CPC was 0.08 ml after 12 months (i.e., 2 vol.%). Although our results did not reach significance, we could demonstrate a high precision (reproducibility) of our method with a mean error estimate of 0.005 ml. We therefore assumed that resorption of the used CPC is very slow and progresses only 500 μm from the initial cement surface after 12 months. In CT, the surface of the cement implant is represented by border voxels containing variable proportions of cement and bone. CT is best suited for in vivo evaluation because of the high signal-to-noise ratio at the bone–cement interface. Thus, we evaluated cement resorption at the bone–cement interface by measuring the density changes of border voxels with CT. Because of the slow CPC resorption, we hypothesized a measurable effect 12 months after kyphoplasty.
We additionally examined the bone–implant interface in a pilot animal experiment to correlate CT measurements with histomorphometry and to study the process of osseous integration of the used cement types.
Materials and methods
Clinical study
Twenty consecutive postmenopausal female patients with 46 osteoporotic VCF at the thoracolumbar spine were enrolled in this study according to the following inclusion criteria: (1) postmenopausal women with primary osteoporosis and one or more painful osteoporotic VCF requiring chronic pain medication; (2) vertebral fractures were present for >12 months, as analyzed by previous X-rays of the patients; (3) the site of worst pain corresponded to the fractured vertebra, as evaluated by thorough clinical examination and imaging studies; (4) kyphoplasty of the fractured vertebra was considered technically feasible by an interdisciplinary team of surgeons, radiologists, and endocrinologists. The exclusion criteria were secondary osteoporosis (e.g., corticosteroid induced), severe degenerative spine alterations, intervertebral disc prolaps with neural compression, and fractures involving the posterior vertebral wall. The mean age was 62 years (±9 years, range 48–82 years). The 46 procedures were performed using either PMMA (KyphX, Kyphon, Sunnyvale, CA, USA) or CPC (Calcibon, Biomet Merck, Darmstadt, Germany). Twenty-eight VCF in 11 patients were filled with CPC and 18 VCF in nine patients with PMMA. The patients were randomly assigned for treatment with PMMA or CPC cement. All patients gave written informed consent prior to kyphoplasty treatment. The study was approved by the local ethical committee. The kyphoplasty procedure was performed using a standardized bilateral transpedicular approach [9, 19].
Computed tomography measurements
Computed tomography (CT) examinations were performed 48 h after kyphoplasty and after 1 year as follow-up (Volume Zoom, Siemens, Erlangen, Germany). All patients were examined with CT using an identical imaging protocol. The standardized protocol included the following parameters: collimation 4×1 mm (four-detector scanner), reconstruction increment 0.5 mm, normalized pitch of 1, field of view 25 cm, 360 mAs, 120 kV, matrix size 512×512 pixels.
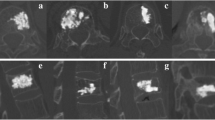
The cement implant is visualized by voxels containing cement material (Fig. 1). Only voxels at the cement surface (i.e., border voxels) contain cement and bone in a variable proportion. The bone–cement proportion of each border voxel determines the density value that can be measured by CT [18]. A reduction of density over a given time period indicates the resorption of cement within the set of border voxels (Ω border ). The border density is defined as:
To measure the cement resorption within all border voxels, we defined the border-voxel density (BVD) as:
where\(\overline{I} ^{{t_{0} }}_{{{\text{border}}}} \) is the border density at time 0 and\(\overline{I} ^{{t_{1} }}_{{{\text{border}}}} \) is the border density at time 1. The BVD represents the relative changes of the signal density of border voxels in %. Thus, the term “density” does not describe properties like “bone mineral density” or “trabecular density” measured in quantitative computed tomography (QCT) or micro-computed tomography (micro-CT) examinations. The CT data were sent to a remote workstation and evaluated using dedicated software algorithms that identify border voxels and computes BVD values automatically to guarantee reproducible measurements (Fig. 1). Data analysis was performed in a blinded fashion without information of the used cement types. We expected no changes in the PMMA group after 12 months because this polymer is not resorbable. PMMA was, therefore, considered as a control and any measured variation in follow-up studies was used for the estimation of precision (reproducibility) [20].
Statistical analysis
Mean values were compared using the t-test. The results are expressed as mean values and standard variation (SD). p-values<0.05 were considered as significant. All calculations were performed with JMP 5.1 (SAS Institute Inc., Cary, NC, USA).
Pilot animal experiment
The animal experiment was conducted in accordance with the guidelines set forth by the local animal protection committee of the regional government. We chose the ovariectomized dog model to be able to implant approximately 1 ml of cement into the vertebral bodies of an osteopenic skeleton, which may, therefore, resemble more closely cement implantation by kyphoplasty in a postmenopausal patient [21]. Osteodensitometry was performed on three lumbar vertebrae utilizing a Hologic 4500 device by sedating the dogs with ketamine. Three 2-year-old foxhounds were ovariectomized (OVX) and lost 3% of their initial bone mass 12 months postovariectomy. A fourth dog was not operated and was maintained as a control with the other animals, but was also fluorochrome-labeled as described below. The control dog was euthanized 12 months postovariectomy. The three OVX dogs received five intravertebral cement implants in general anesthesia, three with CPC (Calcibon, Biomet Merck, Darmstadt, Germany) and two with PMMA (KyphX, Kyphon, Sunnyvale, CA, USA). Thoracic vertebral bodies TH 11 and TH 12 and lumbar vertebra L 1 received CPC, whereas L 2–3 received PMMA. A randomization of the treated vertebrae was not considered as crucial for the evaluation of the histomorphometric findings. The dogs received fluorochrome labeling every three months by intravenous infusion of tetracycline (25 mg/kg), alizarin complexone (30 mg/kg), and calcein green (20 mg/kg). The control dog was labeled during the first 12 months, while the three other dogs were undergoing ovariectomy-induced bone loss. The three OVX dogs were labeled after cement implantation. The three OVX dogs were sacrificed by lethal anesthesia 3, 6, and 12 months after implantation and the control dog 12 months after the other dogs had been ovariectomized (prior to the implantation procedures in the OVX dogs). During lethal anesthesia, the circulating blood was exchanged by perfusion of physiological saline solution, followed by 3% glutaraldehyde containing Indian ink. Thus, blood vessels in osteonal-organized bone are microscopically discernable as black tubes.
The treated vertebrae of the three OVX dogs and the four vertebrae of the control dog were embedded undecalcified into polymethylmethacrylate. Sections were obtained by hard-tissue histology using an Exakt chain saw system (Exakt Vertriebs GmbH, Norderstedt, Germany). The implanted area of each vertebral body (3×5 vertebrae) and the midvertebral volume of the control vertebrae were cut and evaluated. The sections were ground down to a final thickness of 50 μm for histological evaluation.
The marrow star volume of the trabecular bone was measured to verify the osteopenic state 3, 6, and 12 months after cement implantation. The marrow star volume reflects the mean length of lines radiating from a given point in the marrow space until they intersect a trabeculum [22, 23]. Assessment of the marrow star volume was performed in three sections per vertebra (n>100) and is given in μm. The mineral apposition rate (MAR) was assessed as an indicator of the possible effects of the implant on bone formation within the implanted vertebral body. The MAR reflects the distance between two fluorescent labels per labeling period and is given in μm per day [24]. In order to investigate histological responses at the interface, we also measured the contact surface in % of the entire implant’s surface. To determine the extent of contact at the interface, the circumference of the implant was assessed for existing or missing contact to the bone tissue. Three independent investigators counted the number of osteonal structures that were visible within the cement implants of all vertebrae and measured the bone area within the implant area. We did not expect to find any bone tissue within the non-resorbable PMMA implants.
Results
Radiological measurements 12 months after kyphoplasty (clinical study)
All kyphoplasty procedures were performed without perioperative complications. We did not observe changes of blood pressure, pulse frequency, oxygen saturation, or electrolytes perioperatively or during follow-up after 12 months. There was no significant extravasation of cement material into the spinal canal or paravertebral soft tissue.
CPC implants showed a significant decrease of the BVD as compared to PMMA. The mean reduction of the BVD was 4±3.17% (mean±SD, p<0.0006) in the CPC group, compared to 0.08±0.21% in the PMMA group (p>0.05) (Fig. 2). The BVD values ranged from 0.6% to 11% in the CPC group, compared to −0.23% to 0.44% in the PMMA group. The low variation in the PMMA group indicates a high precision (reproducibility) of our CT measurement. We did not observe a radiolucent rim around PMMA or CPC during the 12-month follow-up on the CT images. The clinical outcome after kyphoplasty including incident vertebral fracture rates did not depend on the utilized cement material. In particular, the pain scores (VAS) after kyphoplasty improved by 70% and the mobility score (EVOS) by 25%. There was no significant difference between PMMA and CPC with regards to height restoration, as described in our previous work. Interestingly, we did not observe more new vertebral fractures after kyphoplasty compared to a control group, but, rather, a decreased fracture incidence [5, 6].
Histomorphological observations in dog vertebrae
Three dogs underwent ovariectomy (OVX) 1 year before cement implantation to induce osteopenia and, thus, a state of bone metabolism somewhat similar to the clinical situation of the investigated postmenopausal patients. One dog remained intact, but received fluorochrome labeling. Twelve months after OVX, there was an approximate 3% decrease in bone density compared to the initial bone density, as analyzed by DXA (Hologic 4500, data not shown). To verify the osteopenic state of the OVX dogs by histomorphometry, we determined the mean star volume and observed increasing star volumes in the dogs from 3 to 12 months after cement implantation (Fig. 3). Increasing star volumes are consistent with bone loss at least during the first two years post OVX.
a Marrow star volume reflects the mean length of lines radiating from a given point in the marrow space until they intersect a trabeculum. Assessment of marrow star volume was performed in three sections per vertebra (n>100) and is given in μm. b Columns represent mean values from n=6 vertebrae (+SD) and in the control animal from n=4 vertebrae (+SD). Unpaired, two-tailed t-test, significantly different from control. * p<0.05, ** p<0.01. Bar=200 μm
Next, we examined the possible effects of PMMA and CPC on the bone-forming process within the implanted vertebrae by determination of the mineral apposition rate (MAR). There was no significant difference between the MARs in treated vertebrae (PMMA or CPC). There was also no significant effect on the MARs among the three dogs, indicating no measurable time-dependent effect of the implant materials on the adjacent bone-forming process when compared to the MAR within the vertebrae of the untreated dog (Fig. 4).
a Mineral appositional rate (MAR) reflects the distance between two fluorescent labels per labeling period and is given in μm per day. Green fluorescent labels result from calcein incorporation and red labels from alizarin complexon incorporation. b Each column represents the mean value from one vertebra with n=300 scored fluorescent labels (+SD). Bar=200 μm
The contact surface between the implant and bone was determined as a parameter of osseous integration between bone and the implant. As demonstrated in Fig. 5, CPC exhibited an osseous integration of more than 80% of the entire surface, whereas only 30% of the PMMA implants’ surfaces showed direct bone contact. A thin layer of connective tissue covered 70% of the PMMA implant surface. The remarkable osseous integration of CPC implants occurred as soon as 3 months after implantation and did not change at least until 12 months postimplantation. Therefore, bone tissue grows onto CPC implants almost entirely within the first 3 months after implantation and, thereafter, there is no apparent change with regards to the contact surface. All CPC implants were entirely covered by newly formed bone tissue after 12 months. There was no evidence for the interposition of connective tissue around CPC.
To determine the extent of bone–implant contact at the interface, the circumference of the implant was assessed for contact with bone tissue (red line=no contact, green line=direct contact). Bone implant contact surface of PMMA (a) and CPC (b) implants as a percentage of the entire implant surface 6 months postimplantation. Three sections were scored per vertebra. Bar=200 μm. c Each column represents the mean value in % (+SD). Unpaired, two-tailed t-test, significantly different from CPC. *** p<0.001
Osteonal penetration occurred only at the interface of CPC, but never at the bone–PMMA interface (Fig. 6). There was no bone tissue or osteons discernable within the PMMA implants. The number of osteons within the CPC implants increased from 3 to 12 months post-implantation (12.3±4.9, 45.1±23.6, and 65.6±15, respectively). The time-dependent increase was significant after 6 and 12 months compared to the value at 3 months (p<0.05) (Fig. 7).
a Bone–CPC interface after 12 months, demonstrating the ingrowth of osteonal-organized bone (arrows). Bone lamellae have developed around centrally localized blood vessels that have been perfused by Indian ink prior to sacrifice. Bar=200 μm. b Bone–PMMA interface after 12 months: fibrous layer discernable between PMMA and bone (arrows). Bar=200 μm
We also evaluated the osseous substitution (via osteonal penetration) of the CPC implants by histomorphometry. Figure 8 shows an increase of the measured bone tissue area (osteonal area within the implant) compared to the assessed total area of the implant. Therefore, osseous substitution of the applied CPC implants by autologous bone tissue appears to be initialized by osseous ingrowth via osteonal penetration. Therefore, the total implant area remained constant, while osteonal bone tissue appeared within the CPC implant area, but not within the PMMA implants.
Discussion
Calcium phosphate cements (CPC) are resorbable materials which may qualify as alternative bone substitutes to polymethylmethacrylate (PMMA) in orthopedic surgery and for use in vertebro- or kyphoplasty [10, 11, 15, 16]. Histological evidence from animal experiments suggests that the resorption of CPC in bone tissue occurs by osteoclastic activity [25–27]. Lu et al. reported the resorption of CPC from the periphery to the center [17]. They found different resorption rates of various calcium phosphate biomaterials injected into femoral or tibial condyles in a rabbit model, which may explain differences in the resorption rates of various CPC materials.
In vertebral compression fractures (VCF), where mechanical stability is required, the rates of cement resorption and formation of new bone are crucial to prevent further deformation of the treated and, thereby, buttressed vertebrae. In a previous clinical study, we developed a method for the volumetric measurement of intravertebral cement implants by computed tomography (CT). We found a mean volume reduction of CPC of 0.08 ml after 12 months compared to PMMA, indicating a slow resorption at the CPC surface. However, these findings did not reach significance [18]. In this work, we addressed this phenomenon by analyzing the cement surface via CT by applying dedicated software to detect density changes of border voxels only, which may indicate cement resorption at the bone–cement interface. We calculated the border density of CPC and PMMA as a control and found it to be significantly decreased only in CPC 12 months after kyphoplasty.
We next investigated the histomorphological basis for the observed reduction of the border density in CPC and compared the histology of CPC- and PMMA-treated vertebrae. We chose an animal experiment for the qualitative histomorphological evaluation of the bone–implant interface of CPC and PMMA implants because patients’ biopsies could not be obtained.
First, as demonstrated in Fig. 5, the CPC implants exhibited an osseous integration (i.e., bone coverage) of more than 90% of their entire surface, whereas only 30% of the PMMA implants’ surface had direct contact with bone tissue. A thin layer of connective tissue covered 70% of the PMMA implant surface. The remarkable osseous integration of CPC implants occurred as soon as after 3 months, and did not change until 12 months postimplantation. The difference between CPC and PMMA in the observed osseous integration is not due to the effects of any ingredients of the cement material affecting bone formation, as there was no difference in mineral apposition rates (Fig. 4). These findings are consistent with previous reports demonstrating bone apposition on CPC implants without inflammatory reaction or fibrous encapsulation compared to PMMA [17, 27–29].
Further analysis of the bone–implant interfaces revealed osteonal penetration only of the CPC implants’ surfaces within the first 3 months. Osteonal penetration of bone tissue into the surface of CPC implants is consistent with the radiological observation of a decreased border density of the CPC implants 1 year after CPC implantation (Fig. 2).
The conclusions of our study are limited for various reasons. First, direct comparison of radiological and histological findings was not possible because the animals could not be examined with CT. In addition, the confidence of our CT measurements is dependent on the shape and the size of the cement implant and the resolution of the CT scan. To overcome this drawback, we standardized the study procedure. First, we used an identical imaging protocol. By comparing resorbable CPC with a non-resorbable PMMA as a control, we could demonstrate a significant difference of the border-voxel density (BVD) after a 12-month follow-up. The non-significant change of border density of PMMA implants reflects that the error could be limited to a minimum. In addition, in our pilot animal experiment, the histomorphometric evaluation of only one animal per time point limits a generalization of the histological findings.
In conclusion, the border density at the bone–cement interface can be evaluated in vivo by measuring the density changes of border voxels with CT and a dedicated software analysis package. Resorbable CPC shows a reduction of signal density, whereas non-resorbable PMMA does not change over time. Penetration of blood vessels into the CPC implant followed by osteonal bone formation on the walls of the formed tubules appears to be a possible histological basis for the observed decrease of border density in CPC implants. The histological observations are consistent with the possibility of an osseous substitution of CPC implants after several years. Long-term clinical follow-up by adequate CT technology is required to clarify whether a replacement of CPC implants by autologous bone tissue via osteonal ingrowth does actually occur in patients, e.g., 30 years after kyphoplasty.
References
Peh WC, Gilula LA, Peck DD (2002) Percutaneous vertebroplasty for severe osteoporotic vertebral body compression fractures. Radiology 223(1):121–126
Evans AJ, Jensen ME, Kip KE, DeNardo AJ, Lawler GJ, Negin GA, Remley KB, Boutin SM, Dunnagan SA (2003) Vertebral compression fractures: pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty retrospective report of 245 cases. Radiology 226(2):366–372
Watts NB, Harris ST, Genant HK (2001) Treatment of painful osteoporotic vertebral fractures with percutaneous vertebroplasty or kyphoplasty. Osteoporos Int 12(6):429–437
Heini PF (2005) The current treatment—a survey of osteoporotic fracture treatment. Osteoporotic spine fractures: the spine surgeon’s perspective. Osteoporos Int 16(Suppl 2):S85–S92
Kasperk C, Hillmeier J, Noldge G, Grafe IA, Da Fonseca K, Raupp D, Bardenheuer H, Libicher M, Liegibel UM, Sommer U, Hilscher U, Pyerin W, Vetter M, Meinzer HP, Meeder PJ, Taylor RS, Nawroth P (2005) Treatment of painful vertebral fractures by kyphoplasty in patients with primary osteoporosis: a prospective nonrandomized controlled study. J Bone Miner Res 20(4):604–612
Grafe IA, Da Fonseca K, Hillmeier J, Meeder PJ, Libicher M, Noldge G, Bardenheuer H, Pyerin W, Basler L, Weiss C, Taylor RS, Nawroth P, Kasperk C (2005) Reduction of pain and fracture incidence after kyphoplasty: 1-year outcomes of a prospective controlled trial of patients with primary osteoporosis. Osteoporos Int 16(12):2005–2012
Kallmes DF, Jensen ME (2003) Percutaneous vertebroplasty. Radiology 229(1):27–36
Gangi A, Guth S, Imbert JP, Marin H, Dietemann JL (2003) Percutaneous vertebroplasty: indications, technique, and results. Radiographics 23(2):e10
Lieberman IH, Dudeney S, Reinhardt MK, Bell G (2001) Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine 26(14):1631–1638
Lim TH, Brebach GT, Renner SM, Kim WJ, Kim JG, Lee RE, Andersson GB, An HS (2002) Biomechanical evaluation of an injectable calcium phosphate cement for vertebroplasty. Spine 27(12):1297–1302
Bai B, Jazrawi LM, Kummer FJ, Spivak JM (1999) The use of an injectable, biodegradable calcium phosphate bone substitute for the prophylactic augmentation of osteoporotic vertebrae and the management of vertebral compression fractures. Spine 24(15):1521–1526
Verlaan JJ, van Helden WH, Oner FC, Verbout AJ, Dhert WJ (2002) Balloon vertebroplasty with calcium phosphate cement augmentation for direct restoration of traumatic thoracolumbar vertebral fractures. Spine 27(5):543–548
Nakano M, Hirano N, Matsuura K, Watanabe H, Kitagawa H, Ishihara H, Kawaguchi Y (2002) Percutaneous transpedicular vertebroplasty with calcium phosphate cement in the treatment of osteoporotic vertebral compression and burst fractures. J Neurosurg 97(3 Suppl):287–293
Hillmeier J, Meeder PJ, Noldge G, Kock HJ, Da Fonseca K, Kasperk HC (2004) Balloon kyphoplasty of vertebral compression fractures with a new calcium phosphate cement. Orthopade 33(1):31–39
Tomita S, Molloy S, Jasper LE, Abe M, Belkoff SM (2004) Biomechanical comparison of kyphoplasty with different bone cements. Spine 29(11):1203–1207
Tomita S, Kin A, Yazu M, Abe M (2003) Biomechanical evaluation of kyphoplasty and vertebroplasty with calcium phosphate cement in a simulated osteoporotic compression fracture. J Orthop Sci 8(2):192–197
Lu J, Descamps M, Dejou J, Koubi G, Hardouin P, Lemaitre J, Proust JP (2002) The biodegradation mechanism of calcium phosphate biomaterials in bone. J Biomed Mater Res 63(4):408–412
Libicher M, Vetter M, Wolf, Noeldge G, Kasperk C, Grafe I, Da Fonseca K, Hillmeier J, Meeder PJ, Meinzer HP, Kauffmann GW (2005) CT volumetry of intravertebral cement after kyphoplasty. Comparison of polymethylmethacrylate and calcium phosphate in a 12-month follow-up. Eur Radiol 15(8):1544–1549
Garfin SR, Yuan HA, Reiley MA (2001) New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine 26(14):1511–1515
Schmidt C, Priemel M, Kohler T, Weusten A, Muller R, Amling M, Eckstein F (2003) Precision and accuracy of peripheral quantitative computed tomography (pQCT) in the mouse skeleton compared with histology and microcomputed tomography (microCT). J Bone Miner Res 18(8):1486–1496
Motoie H, Nakamura T, O’Uchi N, Nishikawa H, Kanoh H, Abe T, Kawashima H (1995) Effects of the bisphosphonate YM175 on bone mineral density, strength, structure, and turnover in ovariectomized beagles on concomitant dietary calcium restriction. J Bone Miner Res 10(6):910–920
Croucher PI, Garrahan NJ, Compston JE (1996) Assessment of cancellous bone structure: comparison of strut analysis, trabecular bone pattern factor, and marrow space star volume. J Bone Miner Res 11(7):955–961
Vesterby A, Gundersen HJ, Melsen F (1989) Star volume of marrow space and trabeculae of the first lumbar vertebra: sampling efficiency and biological variation. Bone 10(1):7–13
Lempert UG, Minne HW, Albrecht B, Scharla SH, Matthes F, Ziegler R (1989) 1,25-Dihydroxyvitamin D3 prevents the decrease of bone mineral appositional rate in rats with inflammation-mediated osteopenia (IMO). Bone Miner 7(2):149–158
Yuasa T, Miyamoto Y, Ishikawa K, Takechi M, Nagayama M, Suzuki K (2001) In vitro resorption of three apatite cements with osteoclasts. J Biomed Mater Res 54(3):344–350
Ooms EM, Wolke JG, van de Heuvel MT, Jeschke B, Jansen JA (2003) Histological evaluation of the bone response to calcium phosphate cement implanted in cortical bone. Biomaterials 24(6):989–1000
Ooms EM, Wolke JG, van der Waerden JP, Jansen JA (2002) Trabecular bone response to injectable calcium phosphate (Ca-P) cement. J Biomed Mater Res 61(1):9–18
Verlaan JJ, Oner FC, Slootweg PJ, Verbout AJ, Dhert WJ (2004) Histologic changes after vertebroplasty. J Bone Joint Surg Am 86-A(6):1230–1238
Saito T, Kin Y, Koshino T (2002) Osteogenic response of hydroxyapatite cement implanted into the femur of rats with experimentally induced osteoporosis. Biomaterials 23(13):2711–2716
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Libicher, M., Hillmeier, J., Liegibel, U. et al. Osseous integration of calcium phosphate in osteoporotic vertebral fractures after kyphoplasty: initial results from a clinical and experimental pilot study. Osteoporos Int 17, 1208–1215 (2006). https://doi.org/10.1007/s00198-006-0128-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-006-0128-8