Abstract
Background: More than 1.5 million fractures occur due to osteoporosis each year. This study examines the annual health care utilization and associated expenditures of osteoporotic patients who sustain a new fragility fracture and of those without a new fracture. Methods and procedures: The study sample from commercial claims databases consisted of patients enrolled in US plans between January 1, 1997, and December 31, 2001. Patients with both an osteoporosis diagnosis and a related fracture were classified as “osteoporosis with concurrent fracture”; all other osteoporosis patients were classified as “osteoporosis without concurrent fracture.” Annual utilization and expenditures for the concurrent-fracture cohort were compared with those without concurrent fracture, as well as with a group of patients without osteoporosis (controls) that was matched to the concurrent-fracture cohort based on age, gender, US region, health plan type, and length of enrollment. Exponential conditional mean models were used to compute regression-adjusted total expenditures across the groups. The differences in adjusted expenditures were used to generate the economic burden-of-illness estimates. Results: Osteoporosis patients with concurrent fracture incurred more than twice the overall health care expenditures in the study period, compared with those without fracture (US $15,942 vs $6,476), and nearly three times those of the control group (US $15,942 vs $4,658). Approximately 25% of the overall health care expenditures (US $4,014 of $15,942) for the concurrent-fracture group were osteoporosis-related expenditures, leading to the conclusion that comorbid conditions in osteoporosis patients with concurrent fracture contribute significantly to overall health care costs. Some of these comorbidity-related costs were likely due to pain-related disorders, which occurred significantly more frequently in the concurrent-fracture cohort than in the other groups. Conclusion: Osteoporosis-related expenditures, particularly those related to fracture, were substantial. However, non-osteoporosis-related expenditures to treat comorbid conditions constituted 75% of the overall health care costs in the year after an osteoporosis-related fracture, which warrants further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a systemic skeletal disorder. Formal diagnostic criteria were established in 1994 by the World Health Organization (WHO) Study Group [1]. WHO identifies individuals with osteoporosis as those having a bone mineral density (BMD) score at least 2.5 standard deviations (SD) lower than the young adult normal mean [2]. Severe or established osteoporosis is defined as a BMD of −2.5 SD or lower in the presence of one or more fragility fractures [1, 3].
Osteoporosis is the most common bone disease in the United States, affecting approximately 10 million people. More than 1.5 million osteoporosis-related fractures occur each year. The clinical and humanistic implications of osteoporotic fractures, particularly hip and vertebral fractures, are extensive. In the United States, nearly one third of patients with a hip fracture are admitted to nursing facilities in the year after a fracture, and the incidence mortality rate increases to 20% during this time [4, 5]. Vertebral fractures, although the most frequently occurring type of osteoporosis-related fracture, do not always come to clinical attention and are associated with difficulty in performing activities of daily living, increased bed days, limited activity days, increased physician visits for back pain, and increased risk for future fragility fractures [6, 7, 8, 9].
The treatment and subsequent care required for patients with severe or established osteoporosis places a substantial economic burden on the individual and the health care community as a whole [5, 10, 11, 12]. In a study of the burden of osteoporosis in California during 1998, US $2.4 billion in direct health care costs and over $4 million in lost productivity were attributed to osteoporosis [13]. However, the current literature has not addressed what proportion of these costs are due to treating fractures (e.g., inpatient and ER expenditures), or the proportion of dollars that are spent on osteoporosis medications and BMD testing both before and after fracture. There is little existing literature regarding the economic burden of severe or established osteoporosis in the United States [10, 11, 12].
This study investigates annual direct medical expenditures for a cohort of patients with a diagnosis of osteoporosis immediately after they have sustained an osteoporosis-related fracture, compared with a cohort of patients with an osteoporosis diagnosis and no evidence of fracture, as well as with a control cohort without any osteoporosis diagnoses (matched to the osteoporosis cohort with concurrent fracture). Direct medical costs and utilization are presented by service area for the study populations and are extrapolated to estimate the total direct health care costs of osteoporosis for the entire United States, by fracture status.
Materials and methods
Data source
This retrospective analysis utilized data derived from Medstat’s MarketScan Commercial Claims and Encounters (CCAE) database and the Medicare Supplemental and Coordination of Benefits (COB) database. These databases contain the inpatient, outpatient, and outpatient prescription drug experience of several million employees and their dependents, early retirees, and COBRA continuees (annually), covered under a variety of fee-for-service and capitated health plans. Claims from January 1, 1997, through December 31, 2001, were used for analysis.
Both databases provide detailed cost and utilization data for health care services performed in both inpatient and outpatient settings from approximately 45 large employers, health plans, and government and public organizations throughout the United States. Combined, the databases contain records for approximately 4 million people annually. The CCAE and COB databases are generally representative of the US population in terms of gender (49% male). The age distribution of the CCAE file is heavily weighted toward the 0–64-year age group with a mean age of approximately 34 years, while the COB’s file is most heavily weighted toward the 65+ population with a mean age of 74 years. The regional distribution of both data sets is heavily weighted toward the southern United States and underrepresents the western sections of the country: northeast (12–13%), north central region (22–24%), south region (50–51%), west region (9–14%), and other/unknown (1–3%). MarketScan data have been widely used for outcomes studies in a variety of therapeutic areas [14, 15, 16, 17].
Study population
The osteoporosis study population consisted of patients with a claim for osteoporosis (ICD-9-CM code 733.0x) between January 1, 1998, and December 31, 2000. Identified patients were classified into one of two groups. The osteoporosis with concurrent-fracture cohort consisted of patients with a claim for osteoporosis-related fractures within 6 months of a claim containing an osteoporosis diagnosis code. The osteoporosis-related fracture codes included: pathologic fracture (733.1) or fracture of the vertebral column (805.x) or fracture of the pelvis (808.x) or fracture of the wrist (813.4x, 814.x) or fracture of the femur neck (820.x). Although this is not exhaustive list of osteoporosis-related fractures, these fractures are among the most common and most expensive and were chosen for this reason [4, 5]. The remaining patients with an osteoporosis diagnosis but no claims for any of the above-listed osteoporosis-related fractures are referred to as the osteoporosis without concurrent fracture cohort.
Using this method for case ascertainment of the concurrent-fracture cohort could lead to misclassification. Patients who experience a fracture that is neither vertebral, hip, wrist, nor pathologic, would not be included in the concurrent-fracture cohort. In addition, this study does not capture fractures that occurred prior to 1997. This potential misclassification would result in an underestimation of the prevalence of severe osteoporosis and should lead to a more conservative estimate of health care utilization and expenditures among patients in the concurrent-fracture cohort.
To assess how often a patient may be misclassified as not having osteoporosis but still sustaining one of the fractures of interest, we identified a possible concurrent fracture cohort. These patients were specified as being 55 years of age or older, having sustained one of the specified fractures as well as a prescription for a commonly known treatment for osteoporosis within 6 months of the fracture diagnosis. These patients were identified prior to selecting the control group described below.
A matched sample of randomly selected individuals without a diagnosis of osteoporosis was used as a comparison group. The comparison group was required to have at least 24 months of continuous enrollment at some point between January 1, 1997, and December 31, 2001, and was matched to the concurrent-fracture cohort at a 3:1 ratio using a hierarchical design. The two groups were matched on age, gender, geographic region, and health plan type. If individuals could not be found who matched all criteria, the criteria were reduced in a consistent manner: first by age, then by gender, geographic region, and plan type. Patients with a diagnosis of malignant neoplasm or carcinoma (ICD-9 140.x–208.x, 230.x–239.x), with the exception of melanoma (ICD-9 172.x, 173.x), or Paget’s disease of bone (ICD-9 731.0) at any time during1997–2001 were excluded from all study populations. Figure 1 is a flow chart of patient selection.
This study sought to evaluate osteoporosis patients in all phases of disease progression, from those who were newly diagnosed to those with chronic disease, as well as those who had sustained a fracture due to the disease. The service date of the first observed medical claim with one of the above osteoporosis-related fracture diagnoses was assigned as the index date for the concurrent-fracture cohort. Patients were then required to have 12 months of continuous claims data (enrollment in a health plan) both before and after the index event. For the cohort without concurrent fracture, the service date of the first observed medical claim with an osteoporosis diagnosis after January 1, 1998, was assigned as the index date. No clean period prior to January 1998 was required, and therefore, patients in this cohort may have had a history of osteoporosis prior to their index event. In this way, we attempted to include patients with a history of osteoporosis, not only those who were newly diagnosed with osteoporosis. Again, all qualifying patients were required to have at least 12 months of continuous enrollment following and preceding the index date. Therefore, claims between January 1, 1997, and December 31, 2001, were analyzed in this study.
Outcomes measures and covariates
The primary objective of this study was to quantify the economic burden of illness associated with osteoporosis-related fracture as measured by direct medical expenditures. Annual rates of utilization and expenditures were categorized by service area (hospitalizations, emergency department services, outpatient services, outpatient radiology, and outpatient pharmaceutical prescriptions) in the year before and year following the index date. Claims were further categorized as being osteoporosis-related and non-osteoporosis-related in the two osteoporosis cohorts. Osteoporosis-related claims were those with a coded primary diagnosis of osteoporosis. Additionally, in the concurrent-fracture cohort, claims with one of the specified fracture diagnoses used to classify patients into this cohort also were classified as being osteoporosis-related. The proportion of patients using various osteoporosis-related prescription pharmaceutical agents was assessed. The following agents were considered to be osteoporosis-related drugs: alendronate, risedronate sodium, raloxifene, estrogen and estrogen/progesterone combinations, calcitonin salmon, and disodium etidronate.
The analysis of expenditures was conducted on the subset of patients with fee-for-service insurance coverage during the observation period (83–92% of the study population). Expenditures for patients with fully or partially capitated insurance coverage were not incorporated because the encounter data for these patients often contains data only on patient co-payments and not the amount of physician/facility reimbursements. Inclusion of reported amounts on the encounter data would have required imputations of payments from the fee-for-service sample. It should be noted that actual reimbursed costs and not provider “charges” were contained in the database and form the basis for the expenditure study.
Data were also gathered in the concurrent-fracture cohort to assess rates of fracture, time between osteoporosis diagnosis and initial fracture, time between initial fracture and a second fracture, and use and timing of osteoporosis medications. Patients were considered to have a secondary fracture if they incurred a subsequent claim of the same fracture type 3 months or more after the initially observed fracture. For patients with hip fractures, a claim with a diagnosis for another hip fracture 6 months or more after the initially observed hip fracture was counted as a second distinct hip fracture.
Baseline demographic characteristics for the osteoporosis and nonosteoporosis cohorts included gender, age, insurance plan type, Medicare coverage, and geographic region. Clinical characteristics focused on comorbidity assessments. The 25 most frequently diagnosed comorbidities were identified for each cohort, as well as a Charlson Comorbidity Index (CCI) score [18].
Statistical analyses
The descriptive analyses provided summary statistics on utilization of health care services, key demographic and clinical characteristics for the osteoporosis and nonosteoporosis cohorts. Among patients with fee-for-service insurance coverage, mean annual expenditures for health care services were calculated by service area for each of the groups as well as for the subset of service users (e.g., mean hospital expenditures for patients with at least one hospitalization). Chi-square and t-tests were performed for key covariates of interest; the osteoporosis cohort with concurrent fracture was used as the reference category.
A 12-month expenditure model was estimated. The dependent variable was annual expenditures in the 12-month study period. Confounding factors in the model included presence of fracture, demographic characteristics, geographic location, health plan type, and baseline health characteristics. Baseline health characteristics included the CCI score in the pre-period, other comorbidity indicator variables, and variables describing the presence of osteoporosis-related medications in the pre-period.
In multivariate estimation, outcomes measures may need to be transformed because outliers can skew its distribution. Two-part models in which health care expenditures represent the outcome measure have been estimated with log-transformed US dollars as the outcome or dependent variable [19, 20]. Researchers have routinely retransformed from log-dollars back to dollars to assess mean expenditures through the incorporation of a “smearing” term into the retransformation [21]. Yet, this method is unbiased only if the errors in the equation are unrelated; the case of homoscedastic errors across observations is an uncommon situation in health care expenditures. In our analyses, we have used an alternative strategy by specifying an exponential conditional mean (ECM) model.
Results
The final sample consisted of 4,130 osteoporosis patients with concurrent fractures, 56,878 osteoporosis patients without concurrent fractures, and 12,390 nonosteoporosis (control) patients. Table 1 presents baseline characteristics of the three groups. Patients in the cohort of osteoporosis patients without concurrent fracture were younger, more likely to be female, and have capitated insurance coverage, than patients with concurrent fractures.
Table 2 presents overall health care utilization rates by cohort during the pre-period and study period. Prior to fracture, twice as many patients with concurrent fractures had one or more hospitalizations or ER admissions compared with patients without concurrent fractures and patients in the control cohort (p<0.001). Patients with concurrent fracture also had significantly longer lengths of stay while in the hospital and more admissions or ER visits per person. The two osteoporosis cohorts had similar rates of outpatient visits, radiology services, and pharmaceutical use in the pre-period. Utilization of all types of services was significantly lower in the control cohort as compared to the concurrent-fracture cohort. Between the pre-period and study period, the percentage of patients in the concurrent-fracture cohort with an inpatient event increased by twofold, while the cohort without concurrent fracture had only a 2% increase in inpatient utilization. Overall ER events also increased in the study period for the concurrent-fracture cohort by about 20%. ER utilization increased only slightly in the study period among patients in the other cohorts.
Overall osteoporosis-related utilization increased for both osteoporosis cohorts between the pre-period and study period: almost 50% in the cohort without concurrent fracture and 35% in the concurrent-fracture cohort (Table 3). In the concurrent-fracture cohort, this increase was due to increases in inpatient and ER admissions during the study period which would have included visits due to fractures of interest (hip/pelvis, wrist/arm, vertebrae, or pathologic fractures). The increase in the cohort without concurrent fracture was solely due to claims for an osteoporosis diagnosis and/or pharmaceutical use. With regard to osteoporosis-specific services, the concurrent-fracture cohort was significantly more likely than the cohort without concurrent fracture to incur an outpatient claim with an osteoporosis diagnosis, have osteoporosis-related radiology services, and fill prescriptions for osteoporosis-related pharmaceutical agents. However, the percentage of patients in the cohort without concurrent fracture incurring an outpatient osteoporosis-related visit increased 10-fold from the pre-period to the study period.
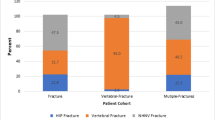
An examination of concurrent fracture in osteoporosis patients (Table 4) revealed that only 62% of patients had a diagnosis of osteoporosis on one or more health care claims in the year prior to fracture, while 38% sustained their fracture before the associated osteoporosis diagnosis. Depending on the type of fracture sustained, between 48% and 57% (results not shown) of these patients had osteoporosis medication use before they sustained an initial fracture; osteoporosis medication use increased to between 63% and 78% after fracture. Hip/pelvis was the most common location for a fracture in this patient population, followed by patients sustaining “multiple fractures,” meaning that they had two or more fractures in different locations on the same claim. The rate of refracture within the year following initial fracture was relatively high—ranging from 13% to 29% depending on initial fracture location.
The distribution of osteoporosis-related medication use in the pre-period and study period by cohort is summarized in Fig. 2. Each category of medication is mutually exclusive: if patients took more than one osteoporosis-related medication in the time period, they were included in the multiple medication category. Multiple drug use could be sequential or concomitant. In the pre-period, the majority of patients in the cohort without concurrent fracture who filled prescriptions for osteoporosis medications used estrogen or estrogen/progesterone combinations only (58%); among medication users in the cohort with concurrent fracture there was more variation in prescription use. The proportion of individuals using osteoporosis medications was higher during the study period for all osteoporosis medications except for those who used estrogen/progesterone only. Osteoporosis patients with concurrent fracture were most likely to be using a combination of therapies in the study period (34% as compared to 25% in the pre-period). Among those in the concurrent-fracture cohort having two or more osteoporosis-related medications during the study period, the following combinations were most common (data not shown): estrogen or estrogen/progesterone combinations and alendronate, 27.5%; calcitonin salmon/alendronate, 18.5%; estrogen or estrogen/progesterone combinations and calcitonin salmon, 12.4%; raloxifene/alendronate, 8.6%; calcitonin salmon/estrogen/alendronate, 6.5%; and calcitonin salmon/raloxifene, 6.2%.
Figure 3 presents data on the regression-adjusted overall and osteoporosis-related expenditures in the pre-period and study period for the three cohorts. Although the pre-period osteoporosis-related expenditures were significantly different, these amounts were similar in magnitude between the two osteoporosis cohorts (US $155 in the cohort without concurrent fracture vs $268 in the cohort with concurrent fracture; p<0.01). Study-period expenditures were more than ten times higher among the concurrent-fracture cohort compared to the cohort without fracture ($4,014 versus $446; p<0.01). Overall health care expenditures in the pre-period were more similar between the control and cohort without concurrent fracture, $4,843 and $4,154, respectively; while the cohort of patients with concurrent fracture had overall pre-period expenditures that were almost twice those of the other two groups ($8,314).
Comorbidities appear to play an important role in the overall health care expenditures attributed to osteoporosis patients. Measures of comorbidity, such as the CCI score, showed that patients in the cohort with concurrent fracture had significantly more comorbidities than those in the other two cohorts; the CCI score for the cohort with concurrent fracture was 2.4 times higher than that for the controls and 1.8 times higher than that for the cohort of osteoporosis patients without concurrent fracture (p<0.01 in both cases). In terms of overall expenditures, only 25% of the concurrent-fracture cohort’s overall total health care expenditures were identified as primarily associated with osteoporosis illness or fracture as indicated by a primary diagnosis on their health care claims (Fig. 3) leaving 75% to be accounted for by other comorbid conditions. Also, 7% of total expenditures were attributed to osteoporosis in the cohort without concurrent fracture, leaving 93% to be accounted for by other comorbid conditions.
A review of the most commonly reported comorbidities during the pre-period and study period found that osteoporosis patients with concurrent fracture were significantly more likely to be diagnosed with pain-related disorders such as arthralgia, chest pain (related to respiratory conditions), backache, abdominal pain, and limb pain than patients without concurrent fracture and patients without osteoporosis. Figure 4 shows that the percentage of patients in the concurrent-fracture cohort diagnosed with these comorbid conditions increased in the study period as well. Figure 5 compares the top 15 most often diagnosed comorbidities among the group with concurrent fracture in the study period compared to the percentage of patients in the other two cohorts who incurred the same diagnoses. In all cases, a higher percentage of patients in the osteoporosis group with fracture incurred the specified conditions even among regular medical diagnoses such as chest pain (ICD-9-CM 786.5), unspecified essential hypertension (401.9), and coronary atherosclerosis (414.0).
Top 15 comorbidities of patients with osteoporosis with concurrent fracture, compared with those with osteoporosis without concurrent fracture and control groups. *The group without concurrent fracture did not have the diagnosis of “congestive heart failure, unspecified” among its top 15 most common comorbidities. **The control group did not have the diagnosis of “other and unspecified disorder of bone and cartilage” among its top 15 comorbidities
The analysis of categorical expenditures revealed that costs associated with hospitalizations and outpatient office visits were the primary drivers of differences between the cohort with concurrent fracture and the two other groups (data not shown). Among the osteoporosis cohort with concurrent fracture we also estimated overall study period expenditures by fracture location using multivariate analysis. Patients sustaining multiple fractures (US $23,274) and hip fractures ($19,973) incurred the highest annual expenditures of the six fracture types investigated. Contrary to common belief, patients with vertebral and pathologic fractures also had large expenditures: $12,529 and $13,405 annually, respectively. Changes in health care expenditures in the year prior to fracture and the year after fracture were also examined. Patients who experienced hip fractures had expenditures in the year after fracture that were nine times higher than the year prior to fracture. Expenditures for patients with multiple fractures (claims for fracture in more than one location on the initial fracture claim) were seven times higher after the fractures, expenditures for wrist fracture patients were five times higher, and expenditures for those with pathologic and vertebral fracture were twice as high as their pre-period costs in the year after fracture.
Discussion
Previous literature has commented on the dearth of empirical studies on the cost of osteoporosis-related fractures [22, 23, 24], although a recent review of the literature reflects a growing cascade of new manuscripts. Recently published studies have examined the cost associated with osteoporosis-related fractures from a population perspective in the United States [13, 25, 26, 27], Sweden [24, 28], France [29], and Belgium [30]. Other studies have examined costs associated with selected fractures such as vertebral fractures and hip fractures [12, 25, 31, 32, 33, 34].
The unique perspective provided by this retrospective claims analysis is the focus on the difference in health care utilization and expenditures of the more severe group of osteoporosis patients who sustained a vertebral, hip, wrist, or pathologic fracture, compared with a cohort of patients diagnosed with osteoporosis without evidence of these same types of osteoporosis-related fractures—as well as to a matched cohort of patients without osteoporosis.
Patients with osteoporosis-related fractures incurred twice the expenditures in the year following fracture, compared with nonfracturing patients diagnosed with osteoporosis, and nearly three times the expenditures of patients without osteoporosis. Regression-adjusted annual costs in the year following fracture were US $14,453 for osteoporosis patients with concurrent fracture, compared with $6,778 and $4,892 in the year following index for osteoporosis patients without concurrent fracture and the matched cohort of patients without osteoporosis, respectively. Osteoporosis-related expenditures were approximately $4,000 per year among patients with fractures, and just $446 annually for patients diagnosed with osteoporosis who did not fracture. With approximately 1.5 million patients in the United States experiencing osteoporosis-related fractures annually, the burden to the US health care system to treat these patients for their fractures and other osteoporosis-related care is estimated to be more than $6 billion using these predicted expenditures. The osteoporosis-related costs to treat the approximately 8.5 million osteoporosis patients in the US health care system without concurrent fractures were estimated to be approximately $3.79 billion annually. These national estimates are slightly lower then those previously reported [25, 26, 34].
Given the dramatic increases in costs for the osteoporosis cohort with concurrent fracture in the year after fracture, some might argue that the differences in cost among the fracturing and nonfracturing cohorts were driven by the services related to treating fractures (osteoporosis-related expenditures). Although this is likely a primary component of the cost differences in the study period, expenditures for patients in the fracturing osteoporosis group were also nearly twice as high as costs for those in the nonfracturing osteoporosis cohort and control cohorts during the 12-month pre-period. Non-osteoporosis-related expenditures comprised most of the expenditures incurred by the concurrent-fracture cohort, suggesting that fracture-related expenditures are only part of the cost story. A body of literature continues to grow about the association between osteoporosis and other chronic diseases such as diabetes mellitus and cardiovascular disease [35, 36, 37, 38]. In addition, a recent study suggests that increased risk of mortality in patients with vertebral fractures may be more a reflection of associated comorbidites [39].
Another comorbid condition that may have accounted for the increased expenditures in the concurrent-fracture group may have been the symptom of pain. Examining the top 15 comorbidities found in the concurrent-fracture cohort in both the pre-study and study periods, arthralgia, respiratory chest pain, backache, and other pain-related diagnoses were among those in the top ten most frequently reported comorbid conditions. The percentage of patients experiencing each of these pain-related comorbidities increased significantly for most diagnoses in the study period (postfracture) as well (Fig. 4). Figure 5 also shows the differences between the cohorts in terms of the most often diagnosed non-osteoporosis-related conditions. Common medical conditions such as hypertension and coronary atherosclerosis appear at a higher rate in the concurrent-fracture group compared with the other two groups.
The use of osteoporosis medications in the concurrent-fracture cohort, both prefracture and postfracture was examined. Approximately 50% of patients with fractures had used one or more osteoporosis medications in the year prior to their fracture. Surprisingly, the proportion of patients with osteoporosis medication use after fracture increased only to 71% of patients. In part, the differential in the effect on total expenditures between the cohorts with and without concurrent fracture may reflect the underutilization of current therapy [40].
Several limitations of this analysis should be considered when interpreting the study conclusions. Although the retrospective data used for this analysis included information that allowed us to identify patients diagnosed with osteoporosis, treatment for fractures, and the presence of comorbid conditions from the diagnosis codes in the medical claims, the data did not contain more detailed clinical information such as bone mineral density results that would allow us to assess disease severity more precisely. Therefore, patients may be misclassified among the osteoporosis study groups, particularly since a limited number of fractures were considered as osteoporosis-related; however, the authors felt that this would lead to a more conservative estimate of osteoporosis-related health care expenditures and utilization due to concurrent fractures. It must be pointed out that some of the increasing costs seen among the group without concurrent fracture may be due to other types of fractures not considered in this study. Additionally, coding of osteoporosis on claims may not be consistent across physicians. To assess the rates at which this might be occurring, we identified older adults (aged greater than 55 years) who had a claim for a fracture and an osteoporosis-related medication but no osteoporosis diagnosis code. Four hundred thirty-seven individuals met this criterion. The results from the analyses of these patients closely mirrored that of the concurrent-fracture cohort. Another limitation to this data is that all patients in the MarketScan databases used in this study had commercial health insurance coverage, and therefore may have health care experiences and expenditures that differ from patients with publicly funded insurance only. Also, only those patients with fee-for-service insurance coverage were used to estimate economic burden of illness. Although this represented the majority of patients with osteoporosis, patients with capitated insurance coverage were slightly younger and had less comorbidity. The potential for sample selection bias due to these issues should be investigated in future research.
In summary, this study confirmed findings from previous research that osteoporosis is a highly prevalent and costly disease [34, 41, 42]. Specifically, whereas osteoporosis patients with concurrent fractures represent just 7% of all osteoporosis patients, they are responsible for 61% of the costs attributable to the disease—or roughly US $6 billion a year. Inpatient and ER expenditures for fracture treatment drive osteoporosis-related expenditures; however, the non-osteoporosis-related expenditures associated with treating comorbid conditions, particularly in the area of pain, accounted for 75% of overall health care costs for the cohort of patients with concurrent fracture. These costs represent a significant contribution to the overall economic burden of treating patients with this condition. Further research should be conducted to better understand the relationship between the risk of osteoporotic fractures and other chronic diseases such as diabetes and cardiovascular disease. In addition, future studies that better delineate the associated cost of symptomatic pain in patients identified with severe osteoporosis are needed.
References
World Health Organization (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO Technical Report Series, No. 843. WHO, Geneva
Buist DSM, LaCroix AZ, Manfredonia D, Abbott T (2002) Identifying postmenopausal women at high risk of fracture in populations: a comparison of three strategies. JAGS 50(6):1031–1038
Weber CE (1998) Uncertainties in bone mineral density T scores. Clin Invest Med 21(2):88–93
National Institute of Arthritis and Musculoskeletal and Skin Diseases (2000) Osteoporosis: progress and promise. National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, MD. http://www.niams.nih.gov/hi/topics/osteoporosis/opbkgr.htm. Cited 26 June 2003
National Institutes of Health (2000) Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Statement. 17(1):1–45
Gehlbach SH, Bigelow C, Heimisdottir M, May S, Walker M, Kirkwood JR (2000) Recognition of Vertebral Fracture in a Clinical Setting. Osteoporos Int 11(7):577–582
Huang C, Ross PD, Wasnich RD (1996) Vertebral fracture and other predictors of physical impairment and health care utilization. Arch Intern Med 156(21):2469–2475
Nevitt MC, Ettinger B, Black DM, Stone K, Jamal SA, Ensrud K, Segal M, Genant HK, Cummings SR (1998) The association of radiographically detected vertebral fractures with back pain and function—a prospective study. Ann Intern Med 128(10):793–800
Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, Licata A, Benhamou L, Geusens P, Flowers K, Stracke H, Seeman E (2001) Risk of new vertebral fracture in the year following a fracture. JAMA 285(3):320–323
Theodorou SJ, Theodorou DJ, Sartoris DJ (2003) Osteoporosis and fractures: the size of the problem. Hosp Med 64(2):87–91
Melton LJ, Gabriel SE, Crowson CS, Tosteson ANA, Johnell O, Kanis JA (2003) Cost-equivalence of different osteoporotic fractures. Osteoporos Int 14(5):383–388
Gabriel SE, Tosteson ANA, Leibson CL, Crowson CS, Pond GR, Hammond CS, Melton LJ (2002) Direct medical costs attributable to osteoporotic fractures. Osteoporos Int 13(4):323–330
Max W, Sinnot P, Kao C, Sung HY, Rice DP (2002) The burden of osteoporosis in California, 1998. Osteoporos Int 13(6):493–500
Berndt ER, A Bir, SH Busch, RG Frank, Normand SL (2002) The medical treatment of depression, 1991–1996: productive inefficiency, expected outcome variations, and price indexes. J Health Econ 21(3):373–396
Bridges CB, Thompson WW, Meltzer MI, Reeve G, Talamonti WJ, Cox NJ, Lilac HA, Hall H, Klimov A, Fukuda K (2000) Effectiveness and cost-benefit of influenza vaccination of healthy working adults: a randomized controlled trial. JAMA 284:1655–1663
Crown WH, Finkelstein S, Berndt ER, Ling D, Poret AW, Rush AJ, Russell JM (2002) The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychol 63(11):963–971
Javitz H., Ward MM, Farber E, Nail L, Vallow SG (2002) The direct cost of care for psoriasis and psoriatic arthritis in the United States. J Am Acad Dermatol 46(6):850–860
D’Hoore W, Bouckaert A, Tilquin C (1996) Practical considerations on the use of the Charlson Comorbidity Index with administrative data bases. J Clin Epidemol 49(12):1429–1433
Manning WG, Newhouse JP, Duan N, Keeler EB, Leibowitz A, Marquis MS (1987) Health insurance and the demand for medical care: evidence from a randomized experiment. Am Econ Rev 77:251–277
Duan N, Manning W, Morris C, Newhouse J (1983) A comparison of alternative models for the demand for medical care. J Business Econ Stat 1(2):115–126
Cameron AC, Trivedi PK (1988). Regression analysis of count data. Cambridge University Press, Cambridge, MA
Zethraeus N, Ben Sedrine W, Caulin F, Corcaud S, Gathon HJ, Haim M, Johnell O, Jönsson B, Kanis JA, Tsouderos Y, Reginster JY (2002) Models for assessing the cost-effectiveness of the treatment and prevention of osteoporosis. Osteoporos Int 13:841–857
Johnell O (2003) Economic implication of osteoporotic spine disease: cost to society. Eur Spine J 12[Suppl 2]:S168–S169
Zethraeus N, Borgström F, Johnell O, Kanis J, Jönsson B (2002) Costs and quality of life associated with osteoporosis related fractures: results from a Swedish survey. Working Paper Series in Economics and Finance, No. 512. http://swopec.hhs.se/hastef/abs/hastef0512.htm. Cited 9 January 2004
Ray NF, Chan JK, Thamer M, Melton LJ (1997) Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res 12:24–35
Hoerger TJ, Downs KE, Lakshmanan MC, Lindrooth RC, Plouffe L, Wendling B, West SL, Ohsfeldt RL (1999) Healthcare use among U.S. women aged 45 and older: total costs and costs for selected postmenopausal health risks. J Womens Health Gend Based Med 8(8):1077–1089
Burge RT, King AB, Balda E, Worley D (2003) Methodology for estimating current and future burden of osteoporosis in state populations: application to Florida in 2000 through 2025. Value Health 6(5):574–583
Jönsson B, Christiansen C, Johnell O, Hedbrandt J, Karlsson G (1996) Cost-effectiveness of fracture prevention in established osteoporosis. Scand J Rheumatol 103[Suppl]:30–38
Levy P, Levy E, Audran M, Cohen-Solal M, Fardellone P, Le Parc JM (2002). The cost of osteoporosis in men: the French situation. Bone 30(4):631–636
Ethgen O, Tellier V, Sedrine WB, DeMaesner J, Gosset C, Reginster JY (2003) Health Related Quality of Life and Cost of ambulatory Care in Osteoporosis: How may such outcome measures be valuable information to health decision makers and papers? Bone 32:718–724
Finnern HW, Sykes DP (2003) The hospital cost of vertebral fractures in the EU: estimates using national datasets. Osteoporos Int 14:429–436
De Laet C, Van Hout BA, Burger H, Weel A, Hofman A, Pols HA (1999) Incremental cost of medical care after hip fracture and first vertebral fracture: the Rotterdam Study. Osteoporos Int 10:66–72
Burge R, Puleo R, Gehlbach S, Worley D, Klar J (2002) Inpatient hospital and post-acute care for vertebral fractures in women. Value Health 5:301–311
Chrischilles E, Shireman T, Wallace R (1994) Costs and health effects of osteoporotic fractures. Bone 15:377–386
Alagiakrishnan K, Juby A, Hanley D, Tymchak W, Sclater A (2003) Role of vascular factors in osteoporosis. J Gerontol A Biol Sci Med Sci 58(4):362–366
Barengolts EI, Berman M, Kukreja SC, Kouznetsova T, Lin C, Chomka EV (1998) Osteoporosis and coronary atherosclerosis in asymptomatic postmenopausal women. Calcif Tissue Int 62:209–213
Hirose K, Tomiyama H, Okazaki R, Arai T, Koji Y, Zaydun G, Hori S, Yamashina A (2003) Increased pulse wave velocity associated with reduced calcaneal quantitative osteo-sono index: possible relationship between atherosclerosis and osteopenia. J Clin Endocrinol Metab 88:2573–2578
Brown SA, Sharpless JL (2004) Osteoporosis: an under-appreciated complication of diabetes. Clin Diabet 22:10–20
Jalava T, Sarna S, Pylkkanen L, Mawer B, Kanis JA, Selby P, Davies M, Adams J, Francis RM, Robinson J, McCloskey E (2003) Association between vertebral fracture and increased mortality in osteoporotic patients. J Bone Miner Res 18:1254–1260
Andrade SE, Majumdar SR, Chan KA, Buist DSM, Go AS, Goodman M, Smith DH, Platt R, Gurwitz JH (2003) Low frequency of treatment of osteoporosis among postmenopausal women following a fracture. Arch Intern Med 163:2052–2057
Gillepspy T, Gillespy MP (1991) Osteoporosis. Radiol Clin North Am 29:77–84
Kanis JA, Pitt FA (1992) Epidemiology of osteoporosis. Bone 13:S7–S15
Acknowledgements
Funding: The authors gratefully acknowledge that funding for this analysis was provided by Eli Lilly and Company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orsini, L.S., Rousculp, M.D., Long, S.R. et al. Health care utilization and expenditures in the United States: a study of osteoporosis-related fractures. Osteoporos Int 16, 359–371 (2005). https://doi.org/10.1007/s00198-004-1694-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-004-1694-2