Abstract
The bisphosphonate ibandronate, administered either daily or intermittently with an extended between-dose interval of >2 months, has been shown to reduce significantly the incidence of vertebral fractures, to increase bone mineral density and to reduce levels of biochemical markers of bone turnover in a phase III randomized study in women with postmenopausal osteoporosis (PMO). Bone histomorphometry was performed on a subgroup of women participating in this study in order to assess bone quality and architecture. The patients were randomized to receive one of the following: placebo, continuous oral daily ibandronate (2.5 mg/day) or intermittent oral ibandronate (20 mg every other day for 12 doses every 3 months). Out of the overall study population of 2,946 patients, 110 were randomly assigned to undergo transiliac bone biopsy at either month 22 or month 34 of treatment. The primary safety endpoint was osteoid thickness in trabecular bone, which was measured to exclude treatment-induced bone mineralization defects. Secondary safety endpoints assessed bone volume, bone turnover and micro-architecture. The primary efficacy endpoint was bone mineralizing surface. In all bone biopsy cores, newly formed trabecular bone retained its structure without any signs of woven bone. Marrow fibrosis and signs of cellular toxicity were not observed. Quantitative assessment demonstrated no impairment in mineralization of bone matrix: osteoid thickness tended to be similar or slightly lower in the ibandronate groups versus the placebo group. All secondary safety variables and the bone efficacy parameter were consistent with the production of normal-quality, newly formed bone and a modest reduction in bone turnover with both ibandronate regimens relative to placebo. Long-term treatment with oral ibandronate, even when administered with an extended between-dose interval of >2 months, produces normal-quality, newly formed bone in women with PMO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone histomorphometry has been used for almost 3 decades in patients with osteoporosis to understand tissue-based bone remodeling abnormalities, to rule out confounding diagnoses and to evaluate the safety and efficacy of pharmaceutical interventions. It has been accompanied more recently by noninvasive diagnostic methods, such as dual-energy X-ray absorptiometry (DEXA) and the measurement of biochemical markers of bone turnover. However, while DEXA accurately measures bone mineral density (BMD) at different individual sites, and biochemical markers give a good indication of the overall balance of bone resorption and formation, neither tool provides information about bone quality or architecture. Histomorphometry is the only technique to do so. In addition, histomorphometric analysis allows assessment of bone turnover at the cellular and tissue level [1]. Consequently, histomorphometry remains an important tool for the evaluation of the long-term effects of therapeutic agents on bone quality and remodeling. Thus, histomorphometric findings are of interest both for the safety and the efficacy of new pharmaceutical agents. The findings reported here are from a study of ibandronate, a new bisphosphonate, in the management of osteoporosis.
Ibandronate is a potent nitrogen-containing bisphosphonate that can be administered with convenient, extended, between-dose intervals of >2 months. Recent results from a multinational, double-blind, placebo-controlled phase III fracture-prevention study (BONE: Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe), conducted in women with postmenopausal osteoporosis (PMO), demonstrated that daily and intermittent regimens of oral ibandronate significantly reduce the incidence of new vertebral fractures by 62% [P=0.0001; 95% confidence interval (CI): 41–75] and 50% (P=0.0006; 95% CI: 26–66), respectively, after 3 years of treatment [2]. This is the first time that a therapeutic agent in the treatment of osteoporosis has prospectively and significantly reduced the risk of fractures when administered with an extended between-dose interval. Both ibandronate regimens also significantly and consistently increased BMD and reduced levels of biochemical markers of turnover over 3 years [2].
To evaluate the effects of these daily and intermittent ibandronate regimens on the quality of newly formed bone and on the bone remodeling process, histological and histomorphometric assessments were performed on transiliac bone biopsies taken from women enrolled in this study. Importantly, this is the first report of the effect of a bisphosphonate given with an extended between-dose interval of >2 months on bone quality and bone remodeling at the trabecular level.
Materials and methods
Bone biopsy study design
Patients participating in the bone biopsy analysis were drawn from a multinational, double-blind, placebo-controlled, randomized phase III fracture-prevention study [2] conducted to assess the efficacy and safety of daily and intermittent oral ibandronate regimens during 3 years of treatment in patients with PMO. In this study, patients were randomized to one of three groups: placebo, continuous oral daily ibandronate (2.5 mg/day) and intermittent oral ibandronate (20 mg every other day for 12 doses every 3 months). Patients in the intermittent group received placebo on days without active treatment, and all patients received daily calcium (500 mg) and vitamin D (400 IU) supplementation.
Study population
A total of 2,946 patients, aged 55–80 years, ≥5 years postmenopause, with one to four prevalent vertebral fractures (T4–L4), and with a BMD T-score of −2.0 to −5.0 in ≥ one vertebra (L1–L4) were enrolled in the phase III antifracture study. Of these 2,946 patients, 110 from 14 centers (13 in North America and one in Europe) were randomly assigned to undergo one transiliac bone biopsy at either month 22 or month 34 of study treatment. Patients were included in the histomorphometry program if separate written informed consent was provided prior to enrolment and if they did not present with any of the following exclusion criteria: allergy or resistance to xylocaine; pathological change in hemostasis or anticoagulant treatment; local skin infection at the biopsy site; Paget’s disease of the iliac bone; contra-indications to tetracycline; previous horizontal transiliac bone biopsies of both iliac crests. Patients were free to withdraw from the histomorphometry program at any time prior to biopsy, without penalty.
Histomorphometry assessments and procedures
Biopsy procedure
At either month 22 or month 34, patients received two doses of tetracycline (1 g/day) over 2 days, given 12 days apart. Horizontal transiliac bone biopsies were taken 3–8 days after the second tetracycline dose using a trephine of 7.5-µm inner diameter. Horizontal transiliac biopsy was selected for this study as it is a well-defined procedure that does not require complex surgery and it allows collection of a representative sample of the ilium [3]. The biopsy cores should be unbroken, i.e., comprise both the inner and outer cortices adjacent to the biopsied trabecular bone, in order to be fully evaluable.
All participating centres adhered to the requirements of the College of American Pathologists’ Laboratory Accreditation Program (Northfield, Illinois, http://www.cap.org).
Qualitative histological analysis
A qualitative histological analysis was performed on all bone biopsies to detect the presence of woven bone, marrow fibrosis, defects in cellular components, absence of tetracycline labels in trabecular or cortical bone, and any other noteworthy features.
Quantitative histomorphometric analysis
Histomorphometry variables were analyzed (masked) in both trabecular and cortical bone by semi-automated image analysis [3]. In complete, unbroken biopsy cores, histomorphometric measurements were performed separately in trabecular and cortical bone; where cores were incomplete or partly broken, measurements were performed only if 20 mm2 of trabecular bone sections (intact cancellous tissue areas) were available for samples containing more than two tetracycline labels, or at least 40 mm2 for samples with less than two tetracycline labels. Sufficient numbers of sections (two or three) of each category (Goldner’s, toluidine-blue, unstained) were obtained at levels at least 300 µm distant from each other to supply 20 or 40 mm2 for analysis. Measurements were performed on Goldner-stained sections for resorption and osteoid variables and on unstained sections for tetracycline-derived variables [3]. Sections were stained with toluidine blue to identify cement lines in measurements of wall thickness. All of the osteons available in the toluidine-blue sections that were clearly marked with cement lines were measured. Measurements were made of the distance between the quiescent surface and the cement line at four randomly placed sites on each osteon. All of the measurements were included in calculating an average value and corrected for obliquity (π/4) to arrive at the final value for mean wall thickness.
All histomorphometric and histological analyses were performed in one laboratory (Creighton University, Omaha, Nebraska) and by the same reader.
Bone biopsy endpoints
Bone mineralization
The primary safety endpoint in the bone biopsy program was osteoid thickness (O.Th, average thickness of the osteoid seams), measured after 2 or 3 years of treatment with the study medication. The thickness of newly formed, osteoblast-derived, unmineralized osteoid matrix provides a reliable assessment of the effects of an agent on the degree of mineralization of the newly formed bone. A substantial increase in O.Th would indicate an impairment of mineralization of the newly formed bone. In contrast, a decrease in O.Th in patients on active drug treatment, relative to placebo, would suggest that a higher proportion of the newly formed bone was mineralized.
Osteoid volume (OV/BV, %), which represents the fraction of bone volume that is non-mineralized osteoid, was also assessed. In addition, a number of other secondary variables were analyzed, including mineral apposition rate (MAR, µm/day, the linear velocity of mineralization of new osteoid as determined by the measure of the distance between the two tetracycline labels as a time marker), adjusted apposition rate (Aj.AR, µm/day, the linear velocity of mineralization of new osteoid adjusted for the fraction of osteoid surface engaged in mineralization) and mineralization lag time (MLT=O.Th/Aj.AR, in days, the mean interval between the deposition of osteoid and its subsequent mineralization).
Slight changes in these variables of bone mineralization are to be expected in association with treatment-induced changes in bone turnover.
Bone volume and turnover
The primary efficacy endpoint for bone remodeling was mineralizing surface (MS), which is the fraction of trabecular surface undergoing bone formation (as judged by the presence of label). The following histomorphometric variables were also calculated to assess bone remodeling: osteoid surface as a percentage of total bone surface (OS/BS, %), bone formation rate (BFR/BS, µm3/m2 per day), the probability that a new cycle of remodeling will be initiated at any point on the trabecular surface [activation frequency (Ac.F), no./year], osteoclast number (NOc, per mm2 of tissue area) and eroded surface (scalloped surface with or without resident osteoclasts; ES/BS, %). When no double tetracycline labels were present in the two or three sections used for static measurements, an extended label search was performed. It consisted of continuing sectioning through the biopsy specimen until double labeling was found. Appositional rate measurements were made on those sites, but because of the rarity of sites in this case, the value for MS/BS was given as zero in the calculations for variables derived from MS/BS.
Trabecular bone micro-architecture and structure
Trabecular thickness (Tb.Th, µm), trabecular number (Tb.N, n/mm) and trabecular separation (Tb.Sp, µm) were measured to assess bone trabecular micro-architecture. These variables are calculated from the cancellous bone area and volume and the length of the bone interface [4]. Wall thickness of trabecular packets (W.Th, µm) and bone volume (BV/TV, %) were also calculated.
Cortical bone
Cortical thickness (Ct.Th, µm) was assessed in order to monitor the effect of ibandronate on the quality of newly formed cortical bone.
Planned sample size
Determination of the sample size for the bone biopsies was based on a predicted decrease in osteoid thickness of 3–4 μm after ibandronate treatment [5]. The standard deviation of the individual decrease could be assumed to exceed the standard deviation for the group effects (SD≈2.5 μm) after 2 years of treatment. A formal, one-sided test of non-inferiority of ibandronate versus placebo required at least 18 patients per treatment group and time point of biopsy, if the tolerance interval was to be fixed at +2.5 μm (wider for an active group versus placebo) for the difference between groups (α=5% and β=10%).
Statistical analysis
To be eligible for analysis, patients must have received study treatment for at least 22 or 34 months and must have been able to provide a bone core at the respective time point. This analysis was based on the intention-to-treat (ITT) population. The analysis of variables was based on exploratory descriptive statistical procedures. Due to the relatively small sample size, results presented here focus on the median values to diminish any distortion from outlying values.
Results
Patient disposition and baseline characteristics
Biopsy cores were obtained in 36, 40 and 34 patients in the placebo group, 2.5 mg daily and 20 mg intermittent groups, respectively. From these patients, 100 biopsy cores were available for quantitative histomorphometric and 110 were available for qualitative histological analysis. The mean time point at which patients underwent the biopsy procedure was either at 22.5 months (range 21.6–25.8 months) or 34.3 months (range 33.4–35.7 months).
Baseline characteristics of the subgroup of patients participating in this histomorphometric analysis were well balanced across the three treatment arms (Table 1). Furthermore, no relevant differences were seen in the biopsy subgroup compared with the overall study population with respect to age, time since menopause, body mass index, ethnic origin, baseline values of BMD or baseline fracture status (data not shown).
Bone biopsy analysis: trabecular bone
Qualitative histological analysis
Tetracycline labels in cortical and trabecular bone were observed in each of the bone biopsy cores obtained. The results of this qualitative histological analysis revealed that in all bone biopsy cores newly formed trabecular bone retained its lamellar structure without any signs of woven bone. No marrow fibrosis or signs of cellular toxicity (bone cells exhibited normal morphology) were observed.
Bone mineralization
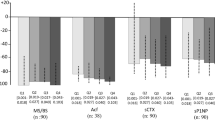
The effects of ibandronate on bone mineralization are presented in Table 2 and Table 3. The primary safety endpoint, osteoid thickness (O.Th), tended to be similar or slightly lower in the ibandronate groups compared with placebo at months 22 and 34. Median values for O.Th by month 22 were slightly reduced from 4.9 μm in the placebo group to 3.9 μm and 4.6 μm in the 2.5 mg and 20 mg groups, respectively. At month 34, the median value for O.Th in the 20 mg group was 5.0 μm, i.e., similar to that of the of the corresponding placebo value of 4.8 μm, whereas the median value in the 2.5 mg group, 4.3 μm, remained slightly below that of the placebo group. The median values for osteoid volume as a percentage of bone volume (OV/BV,%), a secondary safety parameter, tended to be lower in the ibandronate groups compared with placebo. The median MAR was slightly increased in the two ibandronate groups at month 34, while the median Aj.AR was reduced at both time points for both ibandronate groups compared with placebo. With regards to mineralization lag time, MLT, both ibandronate regimens produced higher values at 34 months, compared with placebo.
Bone turnover
The effects of ibandronate on bone turnover are reflected by the variables presented in Tables 4 and 5. The median values for mineralizing surface, MS (the primary efficacy endpoint), in the 2.5 mg (0.7 and 2.0 at months 22 and 34, respectively) and 20 mg (2.2 and 2.1, respectively) ibandronate groups were lower compared with the median values observed in the placebo group (3.6 and 3.1, respectively) at the two time points (Tables 4 and 5). Likewise, the median values for osteoid surface, OS/BS, decreased versus placebo at month 22 and at month 34 in the 2.5 mg ibandronate group. Median activation frequency, Ac.F, showed at least a 50% reduction in the ibandronate groups at month 22 and month 34, relative to placebo. No noticeable difference was observed in osteoclast number, Noc/BS, in either ibandronate group compared with placebo. There were also no noticeable differences in bone formation rate (adjusted to total bone surface), BFR/BS, at either time point, in either ibandronate group relative to placebo.
Variables reflecting the micro-architecture of trabecular bone
Variables reflecting the micro-architecture of spongy bone are trabecular thickness, Tb.Th, trabecular number, Tb.N, and trabecular separation, Tb.Sp (Tables 6 and 7). Median Tb.Th tended to increase at month 34 relative to placebo in both ibandronate groups. Median Tb.Sp tended to decrease and median Tb.N to increase at both time points for the ibandronate regimens versus placebo. None was significant.
As the biopsies that were taken after 22 and 34 months were not paired, a decision was taken to retrospectively analyze the pooled Tb.N and Tb.Sp results (22+34 months) to further assess the effect of ibandronate on these variables within a larger data set. According to this post-hoc analysis, intermittent ibandronate significantly increased Tb.N (P=0.016) and decreased Tb.SP (P=0.016) relative to placebo. No significant changes were seen in patients receiving daily ibandronate relative to placebo.
At month 34, both median wall thickness of trabecular packets, W.Th and bone volume (BV/TV,%) showed increases in both ibandronate groups that were not statistically significant compared with placebo.
Bone biopsy analysis: cortical bone
As expected from the results of previous studies of iliac core biopsies, [6] cortical thickness, Ct.Th, exhibited high variance in all groups (data not shown). Its measurement is subject to large variation as the boundary between cortical and cancellous bone is variable and imprecise.
Discussion
This study is the first to evaluate the effect of daily and intermittent oral ibandronate on bone quality and remodeling in women with PMO. Although the findings are largely confined to trabecular bone, which comprises only 20% of the total skeletal mass, the large surface, higher metabolic activity and greater turnover rate of this compartment make it more sensitive to bisphosphonate treatment than cortical bone. Consequently, histomorphometry is more likely to detect both treatment-related suppression of bone turnover and any potential undesirable effects in trabecular than in cortical bone.
Histomorphometry remains a unique technique for determining the rate of bone turnover at the tissue (trabecular) level and for examining bone quality and architecture. However, it should be recognized that there are limitations associated with this technique. Namely, due to the marked variability of trabecular bone amount at different section levels of the iliac crest, histomorphometry has somewhat limited sensitivity. In addition, the presence of osteoporosis, which is associated with a reduced number of trabeculae, may further reduce the bone surface available for examination. Therefore, for this current analysis, a sufficient number of patients were enrolled in order to ensure sensitivity. In addition, exploratory statistical methods were performed on the median values to remove distortion from outlying values.
A decision was made not to carry out paired biopsies at baseline and at months 22 or 34. This was based on the fact that even from adjacent biopsy sites, intra-individual histomorphometric readings can vary substantially [7, 8, 9]. Additionally, patient acceptance of repetitive transiliac bone biopsies is limited. As paired biopsies were not carried out, it is impossible to compare the findings at 22 and 34 months.
The results from the qualitative histological assessment of all bone biopsy cores demonstrate that newly formed bone is of normal quality following treatment with both daily and intermittent ibandronate. Importantly, no signs of osteomalacia, subtler mineralization defects, marrow fibrosis or cellular toxicity were seen in any of the biopsies.
Likewise, the findings from the quantitative histomorphometric analysis demonstrated no impairment in mineralization of bone matrix: overall, a small decrease in O.Th (primary parameter for safety), a reduction in OV/BV and a slight increase in MAR and MLT were seen with active treatment compared with placebo. Note that, due to a decrease in the rate of bone turnover, a decrease in O.Th and OV/BV and increase in MAR and MLT is expected for anti-resorptive treatments of PMO, but these were not associated with a detrimental effect on bone mineralization.
Overall, mineralizing surfaces and Ac.F were decreased to levels comfortably above zero by ibandronate versus placebo, indicating that inhibition of bone turnover was not excessive. Mineralizing surfaces were reduced by up to 80% and activation frequency was reduced by up to 75% with oral ibandronate versus placebo (reductions seen with daily ibandronate after 22 months). Although there are limitations of comparing data from different trials, these reductions are within the range observed with pamidronate [10], risedronate [11] and zoledronate [12], but are not as great as the effect seen with alendronate in a larger group of patients (n=231) [5].
The moderate reduction in bone turnover seen with oral ibandronate was associated with signs of improved micro-architecture and connectivity as reflected by a slight increase in trabecular bone mass (BV/TV) and a trend towards an increase in Tb.N and Tb.Th and a decrease (both not statistically significant) in Tb.Sp (representing the width and density of trabeculae and distance between trabeculae, respectively). Although not predefined in the data analysis plan, a significant increase in Tb.N and a significant decrease in Tb.SP were observed with intermittent ibandronate relative to placebo when results from 22 and 34 months were pooled (Table 8). This is notable, as any improvements in bone connectivity could lead to improvement in the strength of newly formed bone [13].
These results confirm findings from preclinical studies with ibandronate. For example, histomorphometric assessment demonstrated that bone structure remained normal in ovariohysterectomised dogs treated with daily or intermittent ibandronate for 1 year [14] and in ovariectomized cynomolgus monkeys receiving monthly intravenous ibandronate injections for 16 months [15].
Conclusions
This histomorphometric analysis confirms that long-term treatment with oral ibandronate produces normal-quality newly formed bone, whether administered daily or with an extended between-dose interval. These results support the excellent overall safety profile of ibandronate, the first osteoporosis medication proven to offer lasting antifracture efficacy in a regimen with a between-dose interval >2 months in a prospective trial [2].
References
Frost HM (1969) Tetracycline based histological analysis of bone remodelling. Calcif Tissue Res 3:211–237
Delmas P, Recker R, Stakkestad JA, et al (2002) Oral ibandronate significantly reduces fracture risk in postmenopausal osteoporosis when administered daily or with a unique drug-free interval: results from a pivotal phase III study (abstract). Osteoporos Int 13 [Suppl 1]:S15
Chavassieux PM, Arlot M, Meunier PJ (2001) Clinical use of bone biopsy. In: Marcus R, Feldman D, Kelsey J (eds) Osteoporosis. Academic Press, San Diego, pp 501–509
Parfitt AM, Drezner MK, Glorieux FH, et al (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Chavassieux PM, Arlot ME, Reda C, et al (1997) Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest 100:1475–1480
Compston JE, Vedi S, Stellon AJ (1986) Inter-observer and intra-observer variation in bone histomorphometry. Calcif Tissue Int 38:67–70
Chavassieux PM, Arlot ME, Meunier PJ (1985) Intersample variation in bone histomorphometry: comparison between parameter values measured on two contiguous transiliac bone biopsies. Calcif Tissue Int 37:345–350
de Vernejoul MC, Kuntz D, Miravet L, Goutallier D, Ryckewaert A (1981) Bone histomorphometric reproducibility in normal patients. Calcif Tissue Int 33:369–374
Bergot C, Laval-Jeantet AM, Laval-Jeantet M (1978) Etude critique des variations de la mesure de la masse osseuse par biopsie iliaque. Rev Rhum Mal Osteoartic 45:317–324
Bravenboer N, Papapoulos SE, Holzmann P, Hamdy NA, Netelenbos JC, Lips P (1999) Bone histomorphometric evaluation of pamidronate treatment in clinically manifest osteoporosis. Osteoporos Int 9:489–493
Eriksen EF, Melson F, Sod E, Chines A, Law L (2001) The effect of 5-year risedronate therapy on bone histology and histomorphometry (abstract). J Bone Miner Res 16 [Suppl 1]:S218
Reid IR, Brown JP, Burckhardt P, et al (2002) Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med 346:653–661
Marcus R, Majumdar S (2001) The nature of osteoporosis. In: Marcus R, Feldman D, Kelsey J (eds) Osteoporosis. Academic Press, San Diego, pp 3–17
Monier-Faugere MC, Geng Z, Paschalais E, et al (1999) Intermittent and continuous administration of the bisphosphonate ibandronate in ovariohysterectomized beagle dogs: effects on bone morphometry and mineral properties. J Bone Miner Res 14:1768–1778
Smith SY, Recker RR, Hannan M, Müller R, Bauss F (2003) Intermittent intravenous administration of the bisphosphonate ibandronate prevents bone loss and maintains bone strength and quality in ovariectomized cynomolgus monkeys. Bone 32:45–55
Acknowledgements
This trial was sponsored by F. Hoffmann-La Roche Ltd., Basel, Switzerland. The authors wish to recognize the help of the histomorphometry technicians, Susan Bare and Toni Howard.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Recker, R.R., Weinstein, R.S., Chesnut, C.H. et al. Histomorphometric evaluation of daily and intermittent oral ibandronate in women with postmenopausal osteoporosis: results from the BONE study. Osteoporos Int 15, 231–237 (2004). https://doi.org/10.1007/s00198-003-1530-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-003-1530-0