Abstract
Introduction and hypothesis
Vaginal surface electromyography (sEMG) is commonly used to assess pelvic floor muscle (PFM) function and dysfunction but there is a lack of studies regarding the assessment properties. The aim of the study was to test the hypotheses that sEMG has good test-retest intratester reliability, good criterion validity and is responsive to changes compared to manometry.
Methods
PFM resting tone, maximum voluntary contraction (MVC) and endurance were measured in 66 women with pelvic floor dysfunction. One assessment by manometry was followed by two testing sessions with sEMG at baseline. After 4 to 42 weeks of supervised PFM strength training, 29 participants were retested with both devices.
Results
Median age of the participants was 41 years (range 24–83) and parity 2 (range 0–10). Very good test-retest intratester reliability was found for all three sEMG measurements. The correlation between sEMG and manometry was moderate for vaginal resting tone (r = 0.42, n = 66, p < 0.001) and strong for MVC (r = 0.66, n = 66, p < 0.001) and endurance (r = 0.67, n = 66, p < 0.001). Following the strength training period, participants demonstrated increased MVC and endurance measured with manometry, but not with sEMG. A significant reduction in resting tone was found only with sEMG.
Conclusion
sEMG is reliable and correlates well with manometry. However, sEMG is not as responsive as manometry for changes in PFM MVC and endurance. For measurement of PFM resting tone, sEMG seems more responsive than manometry, but this requires further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Female pelvic floor dysfunction includes urinary and anal incontinence, pelvic organ prolapse, defecatory dysfunction, pain syndromes and sexual dysfunction [1, 2]. The symptoms and conditions negatively affect quality of life and participation in physical activities [1, 2]. Pelvic floor muscles (PFM) training has level 1 evidence and level A recommendation in the treatment of urinary incontinence and in reducing pelvic floor symptoms in women with pelvic organ prolapse [3, 4]. Improved PFM strength and endurance, achieved by high adherence to supervised strength training protocols, is strongly associated with improvements of PFM dysfunction [3,4,5]. However, > 30% of women do not contract their PFM correctly at their first assessment, even after thorough instructions [6]. Therefore, before commencing a PFM training program it is essential to assess the women’s ability to contract the PFM using reliable, valid and responsive tools [7]. Vaginal palpation of the PFMs is a commonly used method to assess PFM function in physiotherapy, but manometry has been found to be more reliable and valid to measure and differentiate muscle strength [7, 8]. Available quantitative methods are manometry, dynamometry, surface (s), wire and needle electromyography (EMG), ultrasonography and MRI, with manometry and sEMG being the most used methods in clinical practice [7].
EMG devices are easy to use, relatively inexpensive, easily accessible and handy in size, and the newest devices have handhold color screens, providing very good concurrent visual feedback regarding the contraction and relaxation capacity of the PFM. Vaginal probes are considered to be more specific than sEMG with adhesive electrodes, but less specific than intramuscular EMG due to variability in electrode placement within the vagina [7, 9]. A specially designed pressure transducer (Camtech AS, Sandvika, Norway) for measuring vaginal resting pressure, PFM strength and endurance has been in use since 1990 in research and clinical practice and has been shown to have good measurement properties. It is found to be reliable, valid and responsive [10,11,12]. Unfortunately, this manometry device is no longer commercially available.
There are several studies on the reliability of PFM sEMG [9, 13,14,15,16,17], but only two have been conducted in women with pelvic floor dysfunction using a vaginal probe [14, 16]. A review from 2017 [18] concluded that there are validity issues with existing sEMG apparatuses, mainly due to cross-talk from adjacent muscles and lack of psychometric and clinometric studies. Pereira et al. [17] explored the relationship between sEMG and manometry in young nulliparous women. To our knowledge, no such study has been undertaken in women with PFM dysfunction. Furthermore, we have not been able to find studies exploring the responsiveness of sEMG. Therefore, the aims of our present study were to test the hypotheses that sEMG has good test-retest intratester reliability and good criterion validity and that it is responsive to changes compared to manometry.
Materials and methods
The study encompasses both a cross-sectional and a longitudinal design. In the cross-sectional part, test-retest intratester reliability of sEMG and criterion validity comparing sEMG to manometry were investigated. Responsiveness analyses were performed based on the longitudinal study. Women with pelvic floor dysfunction were recruited from February 2017 to March 2019 when they were seeking treatment by a women’s health physiotherapist. To be eligible the participants had to be at least 18 years old and able to read and understand Scandinavian languages. Exclusion criteria were untreated urinary tract infection, inability to contract the PFM and unwillingness to be tested vaginally. All participants gave written informed consent to participate. The study was approved by the Norwegian Centre for Research Data (53,092/3/AH). The Norwegian Regional Committees for Medical and Health Research Ethics identified the present project as quality assurance with no further approval requirements.
Procedure
At baseline the participants answered a questionnaire including demographic and socioeconomic variables. The International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-UI SF) [19] was used to assess type, frequency and amount of urinary incontinence and its impact on quality of life. Bowel complaints were assessed by ICIQ bowel questionnaire [20] and was defined as bowel urgency, flatal and/or fecal incontinence. Sexual complaints were assessed by the validated ICIQ sexual matters module [21] and were defined as some feeling of a ruined sexual life, pain or urinary leakage during intercourse, reduced ability to orgasm and/or a feeling of having a loose vagina or lack of PFM control. A sensation of bulging into the vagina is strongly associated with POP at or below the hymenal ring [1]. Having POP symptoms was assessed by the first question of the Pelvic Organ Prolapse Symptom Score questionnaire [22]: “a feeling of vaginal bulge into or out of the vagina.” To avoid any potential influence of bladder fullness on PFM activity, the women were asked to empty their bladder before examination.
All participants were instructed on how to contract the PFM with minimal or no use of abdominal, hip or gluteal muscles during PFM contraction. The ability to perform a correct PFM contraction was confirmed by visual observation of an inward lift of the perineum and vaginal palpation [7, 10, 12]. To ensure valid measurements, only PFM contractions with simultaneous visible inward movement of the perineum/catheter/probe and no use of abdominal, hip or gluteal muscles were considered correct [10, 12]. The participants were instructed to breath in and out and, trying to be as relaxed as possible, to avoid any voluntary PFM activity during the resting period. They received the same instructions for relaxation and contractions when they were tested with manometry and sEMG. The manometer used (Camtech, Norway) has been found to be reliable and valid if used with simultaneous observation of inward movement of the catheter during PFM contraction [10, 12]. Vaginal resting tone, MVC and endurance were assessed once by manometry and twice with sEMG. Each test consisted of three attempts at maximum PFM contractions being held for 10 s. The resting period between the three test sessions was at least 2 min to ensure restitution of the PFM after the three contractions and to set up the next test. The participants were lying in a supine resting position between the tests to ensure that they were stable during the interim period. Twenty-six participants were asked to report how they perceived the two devices.
In addition to the reliability and validity study, a smaller longitudinal responsiveness study was carried out to evaluate whether the two different measurement tools were able to capture changes within each variable. PFM training was offered women with stress urinary incontinence and pelvic organ prolapse. Participants who had performed a treatment period of PFM training were retested with manometry and sEMG at one follow-up visit. The training protocol has shown to be effective to treat urinary incontinence and pelvic organ prolapse symptoms and to increase PFM strength and endurance [4, 5]. With the present responsiveness study the aim was not to determine the effect of PFM training, but to see if two measurement methods captured corresponding changes in the PFM variables. Hence, the length of the interval between the tests was planned to be as variable as possible and varied from 4 to 42 weeks. The daily PFM home training program consisted of three sets of 8–12 close to maximum PFM contractions. Each contraction was held for 8–10 s. The rest period between the sets was at least 2 min. In addition to the home training program the women were offered a voluntary 60-min group PFMT session once a week with a trained physical therapist [4]. The participants were followed up with vaginal manometry testing at least every 4th week. Based on these consultations the number of contractions, holding time and positions were individualized to each woman to ensure a progression in the home training program. A physical therapist performed all tests and was blinded to background variables and to the results of baseline data during the post-test visit.
Outcome measures
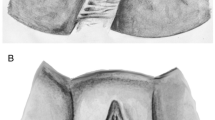
The EMG apparatus NeuroTrac MyoPlus Pro (Quintet, Bergen, Norway) with a 33-mm transverse diameter vaginal probe with two stainless steel lateral electrodes (35 × 15 mm) was used (Periform, Quintet, Bergen, Norway) (Fig. 1). Two participants with PFM pain preferred a smaller probe with a diameter of 25 mm (Anuloform, Quintet, Bergen, Norway). The EMG reference lead wire with an adhesive electrode was placed near the wrist on the right hand. Resting tone was defined as vaginal resting activity and was calculated as the overall average microvolts during the rest periods before and between the PFM contractions. MVC was defined as the average peak activation (μV) during three PFM contractions. PFM endurance was calculated as the work average and consisted of the overall average microvolts achieved during mean muscle activation during the three 10-s PFM contractions. The calculation of endurance and resting tone excludes the first second of each variable to remove the initial spikes of the first contraction attempt and the initial instability of relaxation. Before being digitally sampled, the analogue EMG waveform passed through a 50-Hz notch filter along with a wide filter (18 Hz to 375 Hz). Rectification was automatically achieved digitally by subtracting the average voltage value from each on-going value. Root mean square values were sent 32 times per second to the NeuroTrac MyoPlus Pro and displayed on a full color graphic, touch liquid crystal display screen (Fig. 1).
A manometer from Camtech AS (Sandvika, Norway) with a high precision pressure transducer and a vaginal balloon, sized 67 × 17 mm, was placed about 3.5 cm proximal to the vaginal introitus [10, 12]. Resting tone was defined as vaginal resting pressure and was calculated as the difference between atmospheric pressure and the mean vaginal pressure at rest before and between the PFM contractions (cmH2O). MVC was defined as the difference between the highest measured pressure and the vaginal resting pressure and was calculated as a mean of three MVCs (cmH2O). PFM endurance was defined as a sustained maximal contraction for 10 s and was quantified as the area under the curve (cmH2O/s) [5, 12].
Statistics
Statistical analyses were performed using SPSS 25. Results are given as frequencies and percentages or means with standard deviation (SD), median with minimum and maximum values, or 95% confidence intervals (CI). Preliminary analyses were conducted to ensure no violation of the assumptions of normality, linearity and homoscedasticity. A paired-samples t-test was conducted to calculate the mean difference between test and retest in the reliability and responsiveness analyses. Test-retest intratester reliability was investigated using the intraclass correlation coefficient (ICC 2.1) using a two-way ANOVA for absolute agreement with 95% CI. ICC values between 0.81 and 1.00 were considered very good [23]. Standard error of measurement (SEM) was calculated as: SEM = SDdiff/, where the SDdiff is the SD of the mean difference between test and retest. Smallest detectable change (SDC95%) was calculated using the formula: SDC95% = SEM × 1.96 [24]. To assess responsiveness and validity, the correlation was investigated using Spearman’s rho because of not normally distributed data. A correlation > 0.5 was considered acceptable. The level of significance was set to 0.05 [23]. To visualize the agreement between the two measures, a Bland–Altman plot was used. Since the two measurement instruments had different measurement units, a Z-score was calculated, based on the formula: Z-score (observation value – mean value)/SD. The limits of agreement (LoA) were calculated using the formula: LoA = mean difference ± SDdiff × 1.96. The sample size was determined according to the COSMIN group with at least 50 participants needed for good quality rating [24].
Results
Six participants (9%) were not able to contract the PFM at baseline and thereby excluded from participation. The median age was 41 (range 24–83) years, and median parity was 2 (range 0–10). Background variables including birth history, type of incontinence, bowel and sexual complaints, and mean score on the ICIQ-UI SF are presented in Table 1. The number of participants reporting urinary incontinence was 59 (89.4%), pelvic organ prolapse symptoms 34 (52%), bowel complains 33 (50.0%) and sexual complaints 53 (80.3%).
Reliability study
Of the 66 participants being tested at baseline, 57 were tested twice with sEMG. The reasons for not being tested twice were: crying baby (n = 4), lack of time (n = 3) and empty battery on the EMG (n = 2). Very good test-retest intratester reliability, assessed by ICC2.1, was found for sEMG measurements of resting tone, MVC and PFM endurance (Table 2). The measurement error (SEM%) for sEMG MVC was relatively high (24%) compared to sEMG measurement of endurance (7.5%) (Table 2).
Validity study
Assessments with manometry and sEMG were conducted in 66 participants at baseline. There were no missing values. The resting tone was 29.4 ± 8.6 cmH2O measured by manometry and 7.2 ± 3.7 μV measured by sEMG. For MVC the values were 23.2 ± 16.4 cmH2O and 91.7 ± 68.1 μV and for PFM endurance 165 ± 129 cmH2O/s and 31.6 ± 19.9 μV, respectively. The correlation between manometry and sEMG was moderate for vaginal resting tone (r = 0.42, p < 0.001) and strong for MVC (r = 0.66, p < 0.001) and endurance (r = 0.67, p < 0.001). When comparing manometry MVC with sEMG endurance the correlation was strong (r = 0.64, p < 0.001). The Bland-Altman plots (Fig. 2) showed that women with strong and enduring PFM had greater individual variability compared to women with lower measurements of MVC and endurance.
Bland-Altman plot represents mean (x-axis) and difference (y-axis) between the maximum voluntary contraction (MVC) values (left) and pelvic floor muscle (PFM) endurance measured by sEMG and manometry. Values are based on Z-scores. Limits of agreement (dotted lines) are located at mean difference ± 1.96 standard deviation of the difference
Responsiveness study
Responsiveness analyses were performed in 29 participants returning for a follow-up visit during or after the PFM training period. The time interval between baseline and post-test was 4 to 42 (mean 16.4 ± 9.7) weeks. Changes in vaginal resting tone, MVC and endurance are presented in Table 3. All participants in this sub-study performed PFM training for at least 5 days per week. The participants significantly increased their PFM strength measured with manometry (7.0 cmH2O, 95% CI 4.9–9.2, p < 0.001), but this was not confirmed by sEMG (Table 3). Likewise, manometry captured an increase in PFM endurance by 74 cmH2O/m (95% CI 48–99, p < 0.001), whereas sEMG did not. On the other hand, a significant reduction in resting tone was found measured with sEMG (−1.5 μV, 95% CI -2.7 – -0.3, p = 0.020), but not with manometry. There were no statistically significant correlations between changes in vaginal resting tone, MVC or endurance measured with manometry compared to sEMG (Table 3).
User perspective
Twenty-six participants were asked about how they perceived the two devices. The vast majority found both measurement tools acceptable with no pain or discomfort. However, five participants preferred the manometry device because of less discomfort with a smaller and softer probe. About half of the participants stated that they preferred to be tested with sEMG because of the immediate visualization of the contraction shown on the handheld EMG screen.
Discussion
The present study demonstrated that sEMG had good intratester reliability and correlated well with manometry for measurement of resting tone, MVC and endurance of the PFM. However, compared to manometry sEMG was not responsive to changes in either MVC or endurance. On the other hand, after the training period sEMG showed a reduction in resting tone whereas manometry did not. The results therefore support the hypotheses regarding reliability and validity of sEMG, but not the responsiveness to change of this apparatus.
One sEMG reliability study from 1999 [15] included women with and without pelvic floor dysfunction, but they did not perform recommended statistical reliability analyses [24], so it is difficult to compare the findings to the present study. Koenig [16], however, investigated 50 women with stress urinary incontinence and 20 healthy controls and found good intratester reliability for all measurements. This is in accordance with our results. The measurement errors found in the present study showed that a 16% change in resting tone and 8% change in PFM endurance were needed to detect a real change beyond measurement error (Table 2). This corresponds to the measurement errors found by Koenig [16]. Still, our measurement error for sEMG MVC was high (24%). This implies that MVC is associated with a larger bias than sEMG endurance and sEMG resting tone. Factors such as crosstalk from other muscles, vaginal lubrication, thickness of the vaginal tissue, and movement of the probe and the mucosa can possibly influence the EMG amplitude [25, 26].
The present study found strong correlation between manometry and vaginal sEMG measurements for PFM MVC and endurance. This is better than the moderate correlation found in nulliparous young women [17]. The largest discrepancy between manometry and sEMG was found for women with very good PFM function, scoring high values (Fig. 2). However, this applied to relatively few women and the results support that sEMG is a valid method and can be used safely to assess and teach women how to perform a correct PFM contraction. The present study found a statistically significant correlation between MVC measured with manometry and endurance measured with sEMG. In addition, the measurement errors were highest for sEMG measured MVC and ICC values were best for sEMG endurance (Table 2). Hence, we suggest using sEMG endurance, measured as average work, instead of sEMG MVC when assessing the contraction capacity of the PFM.
We have not been able to find other published studies exploring the responsiveness of sEMG. The increase in MVC and endurance measured by manometry can be used to verify that the PFM training was adequately carried out, even though most of the participants were re-tested in the middle of their treatment period. The training protocol in our study was similar to other protocols that have shown increase in PFM strength and endurance in several RCTs [4, 5]. One RCT [5] found a 44% increase in PFM strength, measured by manometry, 15% increase in muscle thickness, 1.5 cm2 narrowing of the hiatal area and 4–7 mm elevation of the bladder and bowel measured by transperineal ultrasound after 6 months of PFM training. Thicker PFM is associated with higher MVC and PFM endurance [5]. Based on the responsiveness analysis in the present study, sEMG could not be used to detect the same improvements in PFM strength and endurance found by manometry. Our results support recommendations that manometry should be used if the aim is to detect change in PFM strength and endurance [2, 5]. We therefore raise concern about interpretation of results of intervention studies using sEMG to evaluate the effect of PFM training on muscle strength [27, 28].
Although the etiology of painful conditions in the pelvic floor, like vulvodynia, remains poorly understood, increased PFM tone has been proposed as a key pathophysiological mechanism [26, 29]. A review from McLean and Brooks [30] stated a need for interpretation of sEMG in the context of sexual function and dyspareunia. The present study supports that sEMG might be used to assess the effect of relaxation programs [2]. Short-term effect of three consecutive PFM contractions has been found to reduce the immediate resting activity of the PFM measured by sEMG [11]. The present study found indications of a long-term effect of PFM strength training to reduce the PFM resting tone, measured by sEMG. However, manometry did not capture this effect. A possible explanation for this finding may be that the devices measure different aspects of muscle function. If a muscle is relaxed and is short in length, the manometry will measure a high vaginal resting pressure whereas sEMG will measure a low resting activity due to less recruitment of motor units. The result that PFM training might reduce PFM resting tone needs to be further investigated in high-quality studies.
Strengths and limitations
The strengths of the present study are inclusion of a heterogeneous sample of > 50 women with different pelvic floor dysfunctions, mirroring clinical practice, standardization of test procedures, use of validated questionnaires [19,20,21,22] and use of recommended statistical methods [24]. The sample size of > 50 in the cross-sectional study is defined as good by the COSMIN scoring system [24]. The length of the strength training period was not standardized as we wanted variation in gained PFM strength and endurance. The goal was not to determine change in scores, as it would have been for an RCT, but to explore whether the assessment methods captured the same change within each participant. A limitation is that the examiner was not blinded to the results of manometry during the sEMG measurements. However, the examiner was blinded to background variables. In addition, we only tested intratester, not intertester reliability. To capture the day-to-day variation within the subjects the interval between test and retest could have been longer. The responsiveness study was part of the original study design. However, we did not want it to postpone the length of the study period for the reliability and validity study, since these were the main objectives. We therefore ended the study when the sample size for the primary objectives was fulfilled. Hence, a limitation with the responsiveness study is that the sample size was less than the recommended 50 participants [24]. This may affect the generalizability, and the results of the responsiveness analyses should be interpreted with caution.
Conclusion
Vaginal sEMG has good intratester reliability and correlates well with manometry. It can be applied in assessing and teaching women with pelvic floor dysfunction how to contract and relax the PFM. However, it is not as responsive as manometry for changes in MVC and endurance. Manometry may be considered the best measurement tool for clinicians and researchers in measuring change in PFM strength and endurance. However, sEMG can be used in measurement of changes in PFM resting tone, but this requires further investigation.
References
Milsom I, Altman D, Cartwright R, Lapitan MC, Nelson R, Sjöström S, et al. Epidemiology of urinary incontinence (UI) and other lower urinary tract symptoms (LUTS), pelvic organ prolapse (POP) and anal incontinence (AI). In: Abrams PH, Cardoza L, Khoury AE, Wein A, editors. Incontinence volume 1, 6th ed. International consultation on urinary incontinence. Plymbridge United Kingdom: Health Publication Ltd; 2017.
Bø K, Frawley HC, Haylen BT, Abramov Y, Almeida FG, Berghmans B, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Neurourol Urodyn. 2017;36:221–4. https://doi.org/10.1002/nau.23107.
Dumoulin C, Adewuyi T, Booth J, Bradley C, Burgio K, Hagen S, et al. Adult conservative management. In: Abrams PH, Cardoza L, Khoury AE, Wein A, editors. Incontinence volume 2, 6th Ed. International consultation on urinary incontinence. Plymbridge United Kingdom: Health Publication Ltd; 2017. p. 1443–628.
Bø K. Physiotherapy management of urinary incontinence in females. J Physiother. 2020;66:147–54. https://doi.org/10.1016/j.jphys.2020.06.011.
Brækken IH, Majida M, Engh ME, Bø K. Morphological changes after pelvic floor muscle training measured by 3-dimensional ultrasonography: a randomized controlled trial. Obstet Gynecol. 2010;115(2 Pt1):317–24. https://doi.org/10.1097/AOG.0b013e3181cbd35f.
Bump RC, Hurt WG, Fantl JA, Wyman JF. Assessment of Kegel pelvic muscle exercise performance after brief verbal instruction. Am J Obstet Gynecol. 1991;165:322–7.
Bø K, Sherburn M. Evaluation of female pelvic-floor muscle function and strength. Phys Ther. 2005;85:269–82.
Ferreira CH, Barbosa PB, de Oliveira SF, Antônio FI, Franco MM, Bø K. Inter-rater reliability study of the modified Oxford Grading Scale and the Peritron manometer. Physiotherapy. 2011;97:132–8.
Navarro Brazález B, Torres Lacomba M, de la Villa P, Sánchez Sánchez B, Prieto Gómez V, Asúnsolo Del Barco Á, et al. The evaluation of pelvic floor muscle strength in women with pelvic floor dysfunction: a reliability and correlation study. Neurourol Urodynamic. 2018;37:269–77. https://doi.org/10.1002/nau.23287.
Bø K, Kvarstein B, Hagen R, Larsen S. Pelvic floor muscle exercise for the treatment of female stress urinary incontinence: II. Validity of vaginal pressure measurements of pelvic floor muscle strength and the necessity of supplementary methods for control of correct contraction. Neurourol Urodyn. 1990;9:479–87.
Næss I, Bø K. Pelvic floor muscle function in women with provoked vestibulodynia and asymptomatic controls. Int Urogynecol J. 2015;26:1467–73. https://doi.org/10.1007/s00192-015-2660-6.
Tennfjord MK, Engh ME, Bø K. An intra- and interrater reliability and agreement study of vaginal resting pressure, pelvic floor muscle strength, and muscular endurance using a manometer. Int Urogynecol J. 2017;28:1507–14. https://doi.org/10.1007/s00192-017-3290-y.
Auchincloss CC, McLean LJ. The reliability of surface EMG recorded from the pelvic floor muscles. J Neurosci Methods. 2009;30(182):85–96. https://doi.org/10.1016/j.jneumeth.2009.05.027.
Grape HH, Dedering A, Jonasson AF. Retest reliability of surface electromyography on the pelvic floor muscles. Neurourol Urodyn. 2009;28:395–9 doi: 10.1002.
Glazer HI, Romanzi L, Polaneczky M. Pelvic floor muscle surface electromyography. Reliability and clinical predictive validity. J Reproduct Med. 1999;44:779–82.
Koenig I, Luginbuehl H, Radlinger L. Reliability of pelvic floor muscle electromyography tested on healthy women and women with pelvic floor muscle dysfunction. Annals Phys Rehabil Med. 2017;60:382–6. https://doi.org/10.1016/j.rehab.2017.04.002.
Pereira VS, Hirakawa HS, Oliveira AB, Driusso P. Relationship among vaginal palpation, vaginal squeeze pressure, electromyographic and ultrasonographic variables of female pelvic floor muscles. Braz J Phys Ther. 2014;18:428–34. https://doi.org/10.1590/bjpt-rbf.2014.0038.
Flury N, Koenig I, Radlinger L. Crosstalk considerations in studies evaluating pelvic floor muscles using surface electromyography in women: a scoping review. Arch Gynecol Obstet. 2017;295:799–809. https://doi.org/10.1007/s00404-017-4300-5.
Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23:322–30. https://doi.org/10.1002/nau.20041.
Cotterill N, Norton C, Avery KN, Abrams P, Donovan JL. Psychometric evaluation of a new patient-completed questionnaire for evaluating anal incontinence symptoms and impact on quality of life: the ICIQ-B. Dis Colon Rectum. 2011;54:1235–50. https://doi.org/10.1097/DCR.0b013e3182272128.
Jackson S, Donovan J, Brookes S, Eckford S, Swithinbank L, Abrams P. The Bristol female lower urinary tract symptoms questionnaire: development and psychometric testing. Br J Urol. 1996;77:805–12. https://doi.org/10.1046/j.1464-410x.
Hagen S, Glazener C, Sinclair L, Stark D, Bugge C. Psychometric properties of the pelvic organ prolapse symptom score. Br J Obstet Gynaecol. 2009;116:25–31. https://doi.org/10.1111/j.1471-0528.2008.01903.x.
Altman DG. Practical statistics for medical research. London: Chapman & Hall/CRC; 1999.
Terwee CB, Mokkink LB, Knol DL, Ostelo RW, Bouter LM, de Vet HC. Rating the methodological quality in systematic reviews of studies on measurement properties: a scoring system for the COSMIN checklist. Qual Life Res. 2012;21:651–7. https://doi.org/10.1007/s11136-011-9960-1.
Gajdosik RL. Passive extensibility of skeletal muscle: review of the literature with clinical implications. Clin Biomech. 2001;16:87–101.
Morin M, Binik YM, Bourbonnais D, Khalifé S, Ouellet S, Bergeron S. Heightened pelvic floor muscle tone and altered contractility in women with provoked vestibulodynia. J Sex Med. 2017;14:592–600. https://doi.org/10.1016/j.jsxm.2017.
Huebner M, Riegel K, Hinninghofen H, Wallwiener D, Tunn R, Reisenauer C. Pelvic floor muscle training for stress urinary incontinence: a randomized, controlled trial comparing different conservative therapies. Physiother Res Int. 2011;16:133–40.
Oakley SH, Ghodsi VC, Crisp CC, Estanol MV, Westermann LB, Novicki KM, et al. Impact of pelvic floor physical therapy on quality of life and function after obstetric anal sphincter injury: a randomized controlled trial. Female Pelvic Med Reconstruct Surg. 2016;22:205–13. https://doi.org/10.1097/SPV.0000000000000255.
Pukall CF, Goldstein AT, Bergeron S, Foster D, Stein A, Kellogg-Spadt S, et al. Vulvodynia: definition, prevalence, impact, and pathophysiological factors. J Sex Med. 2016;13:291–304. https://doi.org/10.1016/j.jsxm.2015.12.021.
McLean L, Brooks K. What does electromyography tell us about dyspareunia? Sexual Med Rev. 2017;5:282–94. https://doi.org/10.1016/j.sxmr.2017.02.001.
Acknowledgements
We gratefully acknowledge the Norwegian Fund for Post-Graduate Training in Physiotherapy for supporting the project.
Author information
Authors and Affiliations
Contributions
IH Brækken: Protocol/project development, Data Collection, Data analysis, Manuscript writing.
B Stuge: Protocol/project development, Manuscript writing/editing.
AT Tveter: Protocol/project development, Data analysis, Manuscript writing/editing.
K Bø: Protocol/project development, Manuscript writing/editing.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brækken, I.H., Stuge, B., Tveter, A.T. et al. Reliability, validity and responsiveness of pelvic floor muscle surface electromyography and manometry. Int Urogynecol J 32, 3267–3274 (2021). https://doi.org/10.1007/s00192-021-04881-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-021-04881-0