Abstract
Introduction and hypothesis
The objective was to find an alternative treatment to a low-dose antibiotic for the prevention of recurrent urinary tract infections (UTI) and to evaluate the difference in rates of reinfection within 1 year when treated with methenamine hippurate for prophylaxis compared with trimethoprim.
Methods
We present a non-blinded randomized trial comparing methenamine hippurate with trimethoprim for the prevention of recurrent UTI at 12 months after starting treatment. Women over 18 who had at least two culture-positive UTI in the prior 6 months or three in the prior year were included. Ninety-two patients met enrollment criteria and were randomized to receive daily prophylaxis with methenamine hippurate or trimethoprim for a minimum of 6 months. Both intent-to-treat and per-protocol analyses if patients received the alternative drug after randomization were analyzed using Student’s t test, Mann–Whitney U test, Kaplan–Meier curves, log-rank test, and a logistic and multivariate regression model. The primary outcome of this study was culture-proven UTI recurrence by 12 months after initiating prophylaxis.
Results
In the intent-to-treat analysis, we found no difference between groups in recurrent UTI, with a 65% (28 out of 43) recurrence in the trimethoprim group versus 65% (28 out of 43) in the methenamine hippurate group (p = 1.00). In the per-protocol analysis, 65% (26 out of 40) versus 65% (30 out of 46) of patients had UTI recurrences in the trimethoprim group versus the methenamine hippurate group (p = 0.98).
Conclusions
Methenamine hippurate may be an alternative for the prevention of recurrent UTI, with similar rates of recurrence and adverse effects to trimethoprim.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary tract infections (UTIs) are one of the most common bacterial infections in women and account for a significant number of office and emergency department visits per year [1]. UTI symptoms include suprapubic pain, acute dysuria, worsened urinary urgency, frequency and urinary incontinence, fever, and fatigue. Recurrent UTI is defined as two urine culture-proven UTIs within 6 months, or three within 1 year with associated symptoms [2, 3]. The risk of UTI recurrence has been shown to be between 20 and 30% after one infection [4, 5]. One study by Ikäheimo et al. showed that recurrent UTI occurred in 44% of women presenting to a primary care setting who had been treated for a UTI within the prior 12 months [6].
Both antibiotic prophylactic treatment and vaginal estrogen are effective prevention methods for women with recurrent UTI, depending on menopausal status [1, 7,8,9]. Although other prevention treatments such as cranberry, vitamin C, d-mannose, and methenamine hippurate are described, supportive evidence is limited [2]. Antibiotic prophylaxis remains the most effective method of management and is recommended by the American Urological Association, the Canadian Urological Association, and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Surgery [2, 3]. Recommended prophylactic antibiotics include trimethoprim, single-strength trimethoprim/sulfamethoxazole, nitrofurantoin, cephalexin, or fosfomycin [3]. Trimethoprim is prescribed as 100 mg nightly when used for UTI prophylaxis and is covered well by most insurance companies. No difference has been shown between low-dose antibiotic choices [9]. Long-term use of antibiotics, however, can have adverse effects, and incur bacterial resistance [3]. Trimethoprim can lead to Lyell’s syndrome, Stevens–Johnson syndrome and pancytopenia [10]. Even at low doses, long-term nitrofurantoin use may result in hepatotoxicity and pneumonitis, although usually reversible [2].
Methenamine hippurate is an alternative to antibiotic prophylaxis and is a prescription medication with the standard dose of 1 g twice daily. The approximate retail price of a 30-day supply of trimethoprim is $17.25, and is typically covered under a patient’s formulary, compared with a 30-day supply of methenamine hippurate, costing approximately $120, with variable insurance coverage [11]. Methenamine hippurate is a salt that acts as a bacteriostatic agent via formaldehyde production. The last randomized control trial comparing methenamine hippurate with suppressive antibiotics was performed in 1982 [12]. Earlier randomized control trials with methenamine hippurate had promising results showing efficacy without long-term adverse effects. However, these studies had very small populations and did not follow patients for longer than 6 months to 1 year [12, 13]. The most recent Cochrane review analyzed all available methenamine hippurate data prior to June 2012 and found methenamine hippurate to be possibly effective in the prevention of UTI in patients without renal tract abnormalities and when used for short-term prophylaxis [13]. The Cochrane review recommended longer-term, well conducted randomized controlled trials to further clarify this question [13].
Our primary objective was to determine whether prophylaxis using methenamine hippurate is associated with an equal rate of UTI recurrence when compared with trimethoprim. The secondary objective of this study was to determine how well patients were able to tolerate trimethoprim and methenamine hippurate and what adverse effects were observed. We hypothesize that methenamine hippurate will have equal UTI recurrence when compared with antibiotic suppression and a lower side effect profile.
Materials and methods
We present a non-blinded randomized trial comparing the efficacy of methenamine hippurate with that of trimethoprim for the prevention of recurrent UTI at 12 months after starting treatment. A nonblinded study approach was chosen owing to the differences in medication dosages and variable insurance costs between the two medications. Methenamine hippurate was prescribed as 1 g twice daily whereas trimethoprim was prescribed as 100 mg once nightly. The methenamine hippurate pill is much larger than trimethoprim. In addition, the prescriber had to be aware of which medication was to be ordered at the patient’s pharmacy. Patients were enrolled at one tertiary female pelvic medicine and reconstructive surgery practice from June 2016 to May 2018. The study was approved by the local Institutional Review Board (EH16–216) and was registered on clinicaltrials.gov (NCT03077711). Guidelines from the Consolidated Standards of Reporting Trials (CONSORT) were followed.
Eligibility criteria included English-speaking women aged 18 to 99 with a diagnosis of recurrent UTI, having had at least two UTIs in the past 6 months or 3 in the past year that were proven culture-positive of a minimum of 10,000 colony-forming units per milliliter (CFU/ml) [14, 15]. The patients must have had symptoms, in addition to a documented positive urine culture, with any UTI episodes, including acute dysuria, suprapubic pain, fever, worsening urinary urgency, frequency, and urinary incontinence. These same criteria were used when patients called with recurrent UTI episodes after initiating prophylaxis. Women who had received previous prophylaxis for recurrent UTI but had not taken it for a minimum of 30 days were eligible for enrollment. There was a crossover of patients switching randomly assigned groups if a patient experienced adverse effects after starting trial medication, if a drug interaction was discovered after randomization, if a patient was unable to afford one of the trial medications owing to lack a of insurance coverage, or if there was a concern for adverse reactions after reading through the package insert of the trial medication.
Patients were excluded if pregnant or if they had any urinary tract abnormalities, acute pyelonephritis, or renal insufficiency or failure. Further exclusion criteria included known allergy to medications or if a patient was on prophylaxis for post-coital recurrent UTI.
After meeting the inclusion criteria, patients consented to participate in the trial and randomized to prophylaxis with either methenamine hippurate or trimethoprim. The groups were randomized in a 1:1 ratio, methenamine hippurate to trimethoprim. Block randomization was used, with block sizes of four performed by a research coordinator and computer-generated sequence prior to the initiation of the study. Randomization was provided in sequentially numbered, opaque, and sealed envelopes. Aluminum foil inside the envelopes was used to prevent the ability to see through the envelope using intense light. The name, date of birth, and medication randomly assigned to each participant were written on the envelope to prevent subversion of the allocation sequence. Each envelope remained sealed until inclusion and exclusion criteria were met, and informed consent obtained. Patient identification, including initials and date of birth, envelope numbers, and treatment designation were recorded on an enrollment form.
All patients provided written informed consent for trial enrollment. Both attending and fellow physicians in one group practice enrolled participants and the randomly assigned study drug was immediately electronically ordered upon enrollment by the physician who enrolled the patient. The patient was advised to start prophylaxis that day. The only exception was if a patient had acute UTI symptoms upon enrollment; then, a urine culture was obtained and a full course of antibiotics was given, if indicated. Prophylaxis then began after treatment of the acute UTI. Prophylaxis was continued for 6 months after initiation and patients were encouraged to discontinue the medication if they did not develop recurrent UTI, although few desired to continue treatment owing to success with prophylaxis. Patients were followed for the subsequent 6 months for a total of 1 year after enrollment to determine if they developed recurrent UTI.
When patients who enrolled in the study called the office with UTI symptoms, a mid-stream clean catch voided specimen was obtained, and a full course of antibiotics was initiated based on the urine culture results and sensitivity profiles. The prophylactic medication was halted during full-course antibiotic treatment. Subsequent visits were at the start of symptoms of a recurrent UTI if a patient desired to be seen, and at 6 and 12 months after study initiation. Data collected at follow-up visits included UTI recurrence and a urine culture was collected at that time only if indicated by symptoms. Additionally, the number of days from the start of the study to the recurrence of subsequent UTI were recorded. At follow-up visits, patients completed the Morisky Medication Adherence Scale-8 survey, a validated tool used to determine patient compliance with the study medications [16, 17]. The scores correspond to three levels of adherence as follows: <6 is low adherence, 6 to <8 is medium adherence, and 8 is high adherence. This was completed in the first half of the cohort, but was discontinued owing to limited research staff. No other changes were made to the methods after the commencement of the trial.
Based on the results of prior randomized trials comparing methenamine hippurate with trimethoprim, we performed a power and sample size calculation. In one study directly comparing trimethoprim with methenamine hippurate, 10.4% of those in the trimethoprim group had a recurrence, compared with 34.2% in the methenamine hippurate group at 1 year [12]. We determined that 41 patients per arm would be required to detect the expected 23.8% difference in response based on the literature with 80% power at a 0.05 significance level.
The primary outcome of this study was culture-proven UTI recurrence by 12 months after initiating prophylaxis. We used the strictest definition of UTI recurrence, considering even just one recurrent infection in 12 months as overall meeting the definition of recurrent UTI. Secondary outcome measures also included time to subsequent infection from enrollment to the first recurrent UTI and the number of UTI in 1 year after starting prophylaxis. We also compared adverse effects of medications, patient tolerability of medications, and external factors hindering patient use, such as the cost of the study medications. Urine culture data were collected based on the exact number of UTIs prior to enrollment and then at each subsequent UTI after enrollment based on patient symptoms. For each patient, there were at least 2 UTI culture results in 6 months or 3 in 1 year prior to enrollment, in order to meet the definition of recurrent UTI. Recurrent UTI was variable in each group, depending on when a patient felt the symptoms of a UTI.
We conducted an intent-to-treat analysis in addition to a per-protocol analysis to account for potential crossover between medications or if patients had adverse reactions or inability to afford the randomly assigned medication after enrollment. The difference in the recurrence rate between the two groups was determined using Student’s t test, and the differences in the number of recurrences within the study period were compared using a Mann–Whitney U test. We additionally compared the likelihood of recurrence and the number of recurrences using logistic and multivariate linear regression models respectively, to control for baseline risk factors for recurrent UTI. The differences in time to subsequent infection were compared and assessed for statistical significance using Kaplan–Meier survival curves and log-rank tests. Adverse events of the two groups were compared using Student’s t test. All statistical analyses were performed using STATA SE software version 16.0 (StataCorp, College Station, TX, USA). The first author and principal investigator of the study entered the data and the data were validated by the second author.
Results
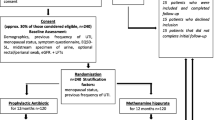
One hundred and four consecutive patients presenting to one urogynecology practice were approached to participate in the study. Ninety-two patients met the eligibility criteria and consented to study participation. Baseline demographic data are shown in Table 1. The mean age of enrolled patients was 71.9 ± 13 years and the mean BMI was 29.5 ± 6.9 kg/m2. Overall, 86 out of 92 patients (93.4%) were post-menopausal and 43 out of 92 of patients (46.7%) were concurrently treated with estrogen vaginal cream, which preceded enrollment into the study. There was no significant difference between groups with regard to age, BMI, parity, reportedly being sexually active, vaginal estrogen use, post-menopausal status, the number of UTIs prior to enrollment, or smoking status. There were no current smokers amongst those enrolled in the trial.
Six patients were omitted from the analytical sample owing to noncompliance with medication adherence or loss to follow-up. A total of 86 patients were included in the final analysis. We performed both intent-to-treat analyses of the data, as well as per-protocol analyses after reassigning patients based on the actual drug taken during the trial. After completing the intent-to-treat analyses, there were 11 patients who were reassigned to the opposing group owing to adverse effects, drug interaction discovered after randomization, inability to afford one of the trial medications owing to lack of insurance coverage, or concern regarding an adverse reaction after reading through the package insert of the trial medication. The CONSORT flow chart of the study protocol is shown in Fig. 1.
Our primary and secondary outcomes are shown in Table 2. Regarding our primary outcome, in the intent-to-treat analysis, 65.1% (28 out of 43) in the trimethoprim group compared with 65.1% (28 out of 43) in the methenamine hippurate group had a symptomatic UTI episode during the follow-up period (p = 1.00). There was no difference in time to subsequent infection between the groups: 100.7 ± 84.4 days in the trimethoprim group compared with 119.3 ± 94.1 days in the methenamine hippurate group (p = 0.52). Both medications significantly decreased the number of UTIs in the year subsequent to enrollment from 4 to 1.5 UTI per year in the trimethoprim compared with 3.7 to 1.6 UTI per year in the methenamine hippurate group (p < 0.01 in either group). In the per-protocol analysis, there was 65% (26 out of 40) recurrence in the trimethoprim group versus 65.2% (30 out of 46) in the methenamine hippurate group (p = 0.98). Both time to subsequent infection and the change in the number of UTIs before and after suppression did not differ from the intent-to-treat analysis (Table 3).
We used multivariate regression to account for baseline UTI risk factors. We used logistic regression when the outcome was whether the patient developed a subsequent infection, and multivariate linear regression when the outcome was the number of subsequent infections that occurred in the study period. Our baseline UTI risk factors included age, the number of UTIs in the prior year, sexual activity, menopausal status, smoking history, duration of treatment prophylaxis, and use of estrogen vaginal cream, which is a known prophylaxis medication used in prevention of recurrent UTI. No variables were found to significantly affect UTI recurrence rates or the number of subsequent infections in either an intent-to-treat or per-protocol analysis (Appendices 1–2). Kaplan–Meier survival curves were used to show differences in time to recurrent UTI after initiation of either treatment. After controlling for baseline UTI risk factors, we found that the risk of developing a recurrent UTI while on prophylaxis was comparable in the two groups, as shown in Fig. 2 for the intent-to-treat analysis and Fig. 3 for the per-protocol analysis. The log-rank tests from the Kaplan–Meier curves were Chi-squared = 0.00 (p = 0.97) for the per-protocol analysis compared with Chi-squared = 0.04 (p = 0.83) for the intent-to-treat analysis.
While on prophylaxis, 10 out of 92 patients (10.9%) experienced an adverse effect that warranted stopping the medication, including diarrhea, rash, and weakness (Table 4). The most common adverse effect reported was diarrhea and this was seen in 1 patient in the trimethoprim group and 2 patients in the methenamine hippurate group. One patient experienced abdominal pain, and one patient had an acute episode of nephrolithiasis thought to be unrelated. One patient developed a Clostridium difficile infection 2 weeks after initiating antibiotic therapy and the medication was discontinued for that reason. In these cases, if patients were willing to try the alternative study medication, they were reassigned to the opposing group. The Morisky Medication Adherence Survey was distributed to 55.4% (51 out of 92) of the cohort, prior to a change in the study methods owing to limited research staff. Scoring corresponded to medium adherence (for scores between 6 and < 8) in both groups, with average scores of 7.3 ± 1.1 in the trimethoprim group compared with 6.9 ± 1.6 in the methenamine hippurate group (p = 0.44). The mean duration of time a patient was recorded taking each study medication was similar in the two groups: 279.5 ± 17.5 days in the trimethoprim group, compared with 272.1 ± 18.4 days in the methenamine group (p = 0.77).
The data on UTI culture results both prior to enrollment and after initiation of prophylactic treatment are reported in Table 5. Each patient had multiple cultures prior to prophylaxis in order to meet the criteria for enrollment, whereas there were variable repeat UTI cultures after initiating treatment based on recurrent UTI symptoms. The most common bacterial strain reported for urine cultures both prior to enrollment and after initiation of prophylaxis was Escherichia coli. E. coli comprised 62.2% of the urine cultures in the trimethoprim group versus 64% of the methenamine group prior to enrollment, and 55.1% of the urine cultures in the trimethoprim group versus 58.1% of the methenamine group after initiation of prophylaxis with any recurrence. Other bacteria reported included Klebsiella pneumoniae, Enterococcus faecalis, extended-spectrum beta-lactamase producing E. coli, Aerococcus urinae, Citrobacter freundii, Actinobaculum schaalii, Raoultella ornithinolytica, Proteus mirabilis, Enterobacter aerogenes, Klebsiella oxytoca, Enterobacter cloacae, methicillin-resistant Staphylococcus aureus, Citrobacter amalonaticus, Pseudomonas aeruginosa, Staphylococcus agalactiae, Citrobacter koseri, Proteus hauseri, and Morganella morganii (Table 5). Overall, antibiotic-resistant bacterial strains were seen in 125 out of 180 (69.4%) of the urine cultures collected at enrollment prior to prophylaxis in the trimethoprim group compared with 126 out of 175 (72%) prior to prophylaxis in the methenamine group. Resistance to even one antibiotic classified a bacterial strain as antibiotic resistant. Bacterial resistance to trimethoprim was similar in the two groups: 52 out of 180 (28.8%) in the trimethoprim group versus 43 out of 175 (24.6%) in the methenamine group. Any antibiotic resistance increased in both groups, with overall resistance being 93.2% (68 out of 73) in the trimethoprim group versus 74.7% (59 out of 79) of the methenamine group. Bacterial strains resistant to trimethoprim were significantly greater in the group taking trimethoprim prophylaxis: 79.5% (58 out of 73) versus 38% (30 out of 79) in the methenamine group (Table 5).
Discussion
In our study, there was a similar proportion of UTI recurrence in the methenamine hippurate compared with the trimethoprim group. There were significant differences in the number of recurrences before and after prophylaxis with both study medications. The time to recurrence in both groups was on average greater than 100 days, which highlights their clinical significance.
The results are promising in that methenamine hippurate may be considered as first-line therapy, does not have the potential to cause the same antibiotic resistance as low-dose antibiotics, and has shown efficacy in preventing recurrent UTI in our study. Additionally, the risk of a serious gastrointestinal infection, Clostridium difficile, although rare in our study and only seen in 1 patient, can be avoided altogether with methenamine hippurate.
Few prior studies have been carried out to compare methenamine hippurate to trimethoprim. One randomized control trial from 1982 by Kasanen et al. allocated patients with recurrent UTI to 1 of 4 groups: placebo, nitrofurantoin 75 mg, methenamine hippurate 1 g, or trimethoprim 100 mg, all given once daily. At 1 year, 63.2% of those in the placebo group had a recurrence, compared with 34.2% in the methenamine hippurate group, 25% in the nitrofurantoin group, and 10.4% in the trimethoprim group [12]. This was the only head-to-head comparison of methenamine hippurate with any suppressive antibiotic found in the literature.
The aim of this study was to determine the difference in prophylaxis comparing trimethoprim to methenamine hippurate in the prevention of recurrent UTI. Owing to significant warranted concerns about antibiotic resistance and a need for antibiotic stewardship in current times, it is imperative to determine an alternative to antibiotics in this vulnerable patient population. Methenamine hippurate has been variably used as an alternative in the prevention of recurrent UTI, but literature supporting its use is outdated.
Our recurrence rate of 65% was higher than those published in the literature, ranging from 20 to 50%, which may indicate the current difficulty in management of this patient population [3]. Our patient population was older (mean age 71) than those of other trials evaluating management of recurrent UTI, but generalizable to patients who are typically seen in a urogynecology practice, usually with an elderly clientele. In addition, more aggressive infections may have been occurring owing to increasing antibiotic resistance in general [3]. We also used a strict definition of having even just one UTI recurrence within a 12-month period to classify a patient as having recurrence after initiating prophylaxis, despite considering this a significant clinical improvement if patients only had that one UTI in the subsequent 12 months. Patients may also have had a lower threshold to calling with UTI symptoms since being enrolled in a clinical trial. Last, medication compliance was variable, possibly resulting in higher recurrence rates as well. The average score of the Morisky Medication Adherence Survey in each group was linked to medium adherence, meaning that on average, patients did not take the medications daily as directed.
Larger scale studies are needed to further answer the question of alternative prophylactic options to suppressive antibiotics. As antibiotic stewardship becomes more of an ethical responsibility to our patients, alternative options must be further investigated. In our trial, higher rates of UTI with bacterial strains resistant to antibiotics were observed in those who took suppressive antibiotics, even at a low dose.
The strengths of this study include its randomized trial design comparing two common treatment regimens and the duration of follow-up. Because inclusion criteria represented a commonly seen population in a female pelvic medicine and reconstructive surgery practice, the results are generalizable. Even though patients on vaginal estrogen at the time of enrollment were included, they were equally distributed between the groups. Patients had a high rate of follow-up and completion of the study protocol.
There are several limitations to this study. All participants elected to participate voluntarily and were not compensated, which resulted in variability in follow-up of participants and management at follow-up visits based on both patient and provider preference. In some cases, prophylaxis was switched owing to one recurrence versus multiple. Patients also chose to continue prophylaxis beyond 6 months if desired, even if doing well. An additional limitation was that our study was not blinded. Investigators had to order the specific medication to the patient’s requested pharmacy. Patients also had to know which medications would be picked up from the pharmacy. The two medications differ in frequency and size and it would have been difficult to blind the subject to these. Last, this study did not include a placebo arm, as it would be unethical to not treat a patient with recurrent UTI, given known treatment options.
Although our trial focused on methenamine hippurate, other options requiring further investigation should include cranberry, d-mannose, probiotics, intravesical antibiotics treatment, vaccination, and hyaluronic acid [10, 18,19,20,21,22,23]. The promising results of our trial, along with those of trials comparing other alternatives are leading us in the right direction.
Conclusion
Our findings support the Cochrane review of methenamine hippurate possibly being an effective prevention strategy, especially in the short term. In addition, methenamine hippurate may be an acceptable long-term prophylaxis alternative in the prevention of recurrent UTI, with similar adverse effects to trimethoprim. A significantly lower number of infections are seen with initiation of either treatment, with greater than 100 days to subsequent UTI after starting treatment.
References
Eells SJ, Bharadwa K, McKinnell JA, Miller LG. Recurrent urinary tract infections among women: comparative effectiveness of 5 prevention and management strategies using a Markov chain Monte Carlo model. Clin Infect Dis. 2014;58(2):147–60.
Abdullatif A, Ahmed K, Zaman I, Khan MS, Dasgupta P. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795–804.
Anger J, Lee U, Ackerman AL, et al. Recurrent uncomplicated urinary tract infections in women: AUA/CUA/SUFU guideline. J Urol. 2019;202(2):282–9.
Geerlings SE, Beerepoot MA, Prins JM. Prevention of recurrent urinary tract infections in women: antimicrobial and nonantimicrobial strategies. Infect Dis Clin N Am. 2014;28(1):135–47.
Mody L, Juthani-Mehta M. JAMA patient page. Urinary tract infections in older women. JAMA. 2014;311(8):874.
Ikäheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;22(1):91–9.
Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753–6.
Eriksen B. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am J Obstet Gynecol. 1999;180(5):1072–9.
Albert X, Huertas I, Pereiró II, Sanfélix J, Gosalbes V, Perrota C. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;3:CD001209.
Shepherd AK, Pottinger PS. Management of urinary tract infections in the era of increasing antimicrobial resistance. Med Clin North Am. 2013;97(4):737–57 xii.
Epocrates. Search drugs: methenamine hippurate and trimethoprim. In Epocrates medical references (Version 21.4.1) [Mobile application software] 2020. Retrieved from http://itunes.apple.com.
Kasanen A, Junnila SY, Kaarsalo E, Hajba A, Sundquist H. Secondary prevention of recurrent urinary tract infections. Comparison of the effect of placebo, methenamine hippurate, nitrofurantoin and trimethoprim alone. Scand J Infect Dis. 1982;14(4):293–6.
Lee BS, Bhuta T, Simpson JM, Craig JC. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;17:10.
EAU Guidelines. Presented at the EAU Annual Congress Amsterdam the Netherlands 2020
Epp A, Larochelle A, Urogynaecology Committee; Family Physicians Advisory Committee. Recurrent urinary tract infection. J Obstet Gynaecol Can. 2010;32(11):1082–101.
Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74.
Moon SJ, Lee WY, Hwang JS, Hong YP, Morisky DE. Accuracy of a screening tool for medication adherence: a systematic review and meta-analysis of the Morisky medication adherence Scale-8. PLoS One. 2017;12(11):e0187139.
Kolman KB. Cystitis and pyelonephritis: diagnosis, treatment, and prevention. Prim Care. 2019;46(2):191–202.
Pietropaolo A, Jones P, Moors M, Birch B, Somani BK. Effectiveness of antimicrobial Intravesical treatment for prophylaxis and treatment of recurrent urinary tract infections (UTI): a systematic review. Curr Urol Rep. 2018;19(10):78.
Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10:CD001321.
Schwenger EM, Tejani AM, Loewen PS. Probiotics for preventing urinary tract infections in adults and children. Cochrane Database Syst Rev. 2015;12:CD008772.
Kranjčec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol. 2014;32(1):79–84.
Yang B, Foley S. First experience in the UK of treating women with recurrent urinary tract infections with the bacterial vaccine Uromune®. BJU Int. 2018;121(2):289–92.
Funding
There was no source of financial support for this study.
Author information
Authors and Affiliations
Contributions
C. Botros: protocol/project development, data collection and management, data analysis, manuscript writing/editing; S. Lozo: data collection and management, manuscript editing; S. Iyer: protocol/project development, data collection, manuscript editing; A. Warren: data management, manuscript writing/editing; R. Goldberg: protocol/project development, data collection, manuscript editing; J. Tomezsko: protocol/project development, data collection, manuscript editing; K. Sasso: data collection and management, manuscript editing; P. Sand: protocol/project development, data collection, manuscript editing; A. Gafni-Kane: protocol/project development, data collection, manuscript editing; A. Biener: data analysis, manuscript editing; S. Botros-Brey: protocol/project development, data collection and management, manuscript writing/editing.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
Appendix 2
Rights and permissions
About this article
Cite this article
Botros, C., Lozo, S., Iyer, S. et al. Methenamine hippurate compared with trimethoprim for the prevention of recurrent urinary tract infections: a randomized clinical trial. Int Urogynecol J 33, 571–580 (2022). https://doi.org/10.1007/s00192-021-04849-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-021-04849-0