Abstract
Introduction and hypothesis
Defecatory dysfunction is a relatively common and challenging problem among women and one that practicing pelvic reconstructive surgeons and gynecologists deal with frequently. A subset of defecatory dysfunction includes obstructed defecation, which can have multiple causes, one of which is descending perineum syndrome (DPS).
Methods
A literature search was performed to identify the pathophysiology, diagnosis, and management of DPS.
Results
Although DPS has been described in the literature for many decades, it is still uncommonly diagnosed and difficult to manage. A high index of suspicion combined with physical examination consistent with excess perineal descent, patient symptom assessment, and imaging in the form of defecography are required for the diagnosis to be accurately made. Primary management options of DPS include conservative measures consisting of bowel regimens and biofeedback. Although various surgical approaches have been described in limited case series, no compelling evidence can be demonstrated at this point to support surgical intervention.
Conclusions
Knowledge of DPS is essential for the practicing pelvic reconstructive surgeon to make a timely diagnosis, avoid harmful treatments, and initiate therapy early on.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Descending perineum syndrome (DPS) is an uncommonly discussed condition associated with obstructed defecation. It is described as increased bulging of the perineum with straining, although perineal descent can also be seen at rest. It was first illustrated by Porter in 1960 in a woman with a history of chronic constipation noted to have a perineal bulge on Valsalva [1]. Several years later, it was described as a discrete clinical syndrome noted in patients with a history of constant straining during defecation [2, 3]. DPS is likely overlooked as a cause of obstructed defecation and is similarly under-represented in the current literature. The aim of this narrative review is to examine the existing literature on the presentation, pathophysiology, diagnosis, and management of DPS. This information can be important for clinicians in allowing them to initiate the proper diagnostic tests and treatment modalities to optimize patient outcomes.
Materials and methods
We conducted a search using PubMed for English-language articles published from January 1960 to January 2015 using the following search terms: descending perineum syndrome, descending perineal syndrome, perineal descent syndrome, and obstructed defecation. Additional relevant publications were selected from the reference list of identified articles. Intervention studies and noncomparative studies were included. Articles were screened by title and abstracts by the authors. A total of 519 publications were identified in PubMed. After duplicate citations were removed, 468 publications remained. Screening based on titles and abstracts for relevance and applicability led to 393 citations being excluded. The remaining 75 articles were reviewed. A total of 25 articles were included (Fig. 1). Owing to the heterogeneity of the study design and results of articles related to treatment and outcomes of DPS, a quantitative analysis could not be performed.
Definition
Although DPS is clearly described by its name, the precise definition varies in the literature based on either physical examination or defecography. Radiologically, perineal descent is described as the craniocaudal movement of the anorectal junction during straining from a fixed point of reference, which may include the ischial tuberosities (bi-ischiatic line), the tip of the coccyx, or more commonly, a line drawn from the coccyx to the pubic symphysis (pubococcygeal line). What constitutes normal versus abnormal perineal descent varies in the literature as there appears to be an overlap of normal and abnormal values. Early defecography studies of women without any bowel symptoms have shown that 77 % had a measured perineal descent of fewer than 3 cm, with another study showing that 84 % of women had descent of fewer than 2 cm [4, 5]. A mean value of 2.6 cm has been seen in women without functional bowel symptoms, with descent up to 4.4 cm also being observed in normal women [6, 7]. Resting perineal descent values ranging from 3 to 4 cm and straining values from 2.5 to 4 cm have all been described as abnormal and suggestive of DPS [2, 8–12]. The heterogeneity of study results underscores the challenge in the ability of imaging to detect anatomical abnormality due to the wide variation of normal values described in the literature.
Signs and symptoms
Patients often present with a history of chronic straining during defecation and the sensation of incomplete evacuation of the rectum followed by a sensation of obstruction. Complaints of mucoid discharge, bleeding, perineal irritation, chronic anal pain, and perineal pruritus are also not uncommon because of the prolapse of the anterior rectal wall [2, 12, 13]. When prompted, patients may admit to the use of splinting and digitation to aid in evacuation [9]. Anecdotally, patients may complain of feeling as though they have a “rock” or “ball” in the pelvic area. Anal incontinence is a potentially long-term sequela in these patients secondary to pudendal neuropathy as a consequence of increased stretching associated with persistent straining [14]. However, this is not a consistent finding if sphincter pressures remain within normal ranges [15–19].
Pathophysiology
Normal defecation is a complex process involving voluntary and involuntary processes in four distinct phases: the basal phase, the predefecatory phase, which generates the urge to defecate, expulsive phase, and the termination of defecation [20]. DPS entails a “vicious cycle” of straining and constipation, which leads to more straining and exacerbation of the anatomical abnormality and descent (Fig. 2). Chronic repetitive straining in the setting of weakened pelvic floor musculature is attributable to a variety of causes, although pregnancy and parturition are thought to be primary contributing factors [21–23]. Overall, risk factors for increased perineal descent appear to be related to female gender, age, vaginal parity, rectocele size, and rectal intussusception [22, 23]. The constipation/chronic repetitive straining results in subsequent perineal descent and ballooning of the rectum with downward projection of the anterior rectal wall into the anal canal and caudally outward into the perineum, which leads to a pronounced feeling of inadequate emptying. This leads to further straining, which can potentially result in the rectal mucosa prolapsing, causing some of the earlier noted symptoms of mucoid discharge, bleeding, and peri-anal pruritus [15].
With excess perineal descent, pudendal neuropathy is a theoretical sequela. It has been estimated that with persistent straining, pudendal nerve stretching of approximately 20 % of its length can occur [24, 25]. Animal models have shown that stretching of peripheral nerves consisting of only 12 % elongation can cause permanent damage [26]. The theory of pudendal strain leading to anal incontinence is conflicting as some studies indicate a linear relationship between increased pudendal nerve motor terminal latency (PNMTL) values and increased perineal descent, while others show no such relationship [27–30]. The lack of a consistent association can be potentially attributed to the different and nonstandardized methods in which DPS was diagnosed in each study.
Epidemiology
An accurate estimate of the prevalence of descending perineum syndrome is difficult to make. One study of 158 consecutive patients from a colorectal clinic estimated that 11 % of their patients had measurable perineal descent at either rest or straining, although this study is significantly limited by its patient population and the use of a perineometer to measure distance [21]. Interestingly, using the device has been shown to underestimate perineal descent by nearly 60 % compared with radiography [31]. A retrospective review of 2,816 defecography studies revealed that 9 % studies showed increased perineal descent, although the authors caution that increased perineal descent was not consistently reported and is likely higher [32].
A cross-sectional survey of obstructed defecation symptoms in middle-aged women found that approximately 60 % had experienced some type of obstructed defecation in the past 12 months and that 12 % experienced these symptoms on a weekly or daily basis [33]. What can be inferred based on the available literature is that DPS is likely under-reported and under-diagnosed.
Diagnosis
In the current setting, diagnosis of DPS depends on defecography and correlation with patient signs and symptoms. Briefly, defecography consists of placement of barium paste in the rectum and vagina with subsequent positioning of the patient either on a special commode or in the left lateral decubitus position. Placement of patients in the left lateral decubitus position does not appear to affect perineal descent on straining, but has been shown to minimize perineal descent measurements at rest [11]. Contemporary defecography can use video to capture the entire sequence. During the procedure several images are captured for evaluation and include:
-
1.
At rest with the anal bulb filled
-
2.
During maximum contraction of the anal sphincters and pelvic floor muscles
-
3.
During straining without evacuation
-
4.
During evacuation
-
5.
When evacuation is complete
The two most significant measurements consist of the anorectal angle and movement of the anorectal junction during straining. Variation exists regarding measurement of the anorectal angle as the point of reference used in measuring perineal descent such as the ischial tuberosities, the tip of the coccyx, and the pubococcygeal line have been described in the literature [8, 10]. It is important for the ordering practitioner to be aware of how the procedure is performed at their institution to ensure consistent interpretation. In addition, it is important for the provider to properly counsel patients ahead of time on the nature of defecography to avoid potential anxiety as the examination should simulate physiological conditions as closely as possible to ensure that accurate results are obtained. Defecography is a vital test in the diagnosis of DPS and one must take into account the normal range of perineal descent to appropriately diagnose DPS (Figs. 3, 4).
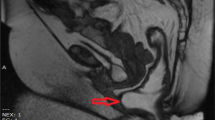
Although contemporary defecography is considered the modality of choice in the evaluation of DPS, magnetic resonance (MR) defecography (also known as dynamic MRI) is a relatively newer imaging technique that shows promise (Figs. 5, 6) [34–36].
One major benefit of MR defecography is the lack of patient exposure to ionizing radiation. Prior research has shown that contemporary defecography exposes the ovaries and uterus to considerable amounts of ionizing radiation [37]. While the absolute amount of radiation is not life-threatening, it does limit the ability to repeat the test if needed. MR defecography also allows for better delineation of the soft tissue structures of multiple compartments of the pelvis, allowing for a broader overview of other potential pelvic floor defects [34–36].
Limitations of MR defecography include the inability to use this modality in patients with certain metal implants. In addition, most MR configurations in the clinical setting comprise closed configuration systems that require the patient to be supine with knees flexed. One prior study compared MR defecography done in patients in the supine and in the sitting position. Although the sensitivity of diagnosis of perineal descent was better in the sitting position compared with the supine position, this difference was not significant. Other pelvic floor disorders, such as rectal intussusception, which could potentially contribute to obstructed defecation symptoms, were more likely to be missed in the supine position [38].
Several studies have compared contemporary defecography with MR defecography, with evidence generally suggesting that contemporary defecography might be better able to measure perineal descent, although others have shown no significant differences [39–42]. One small study of 15 patients, 2 of whom had DPS, noted that contemporary defecography missed one abnormality compared with MR defecography [43].
Other important factors for the ordering provider associated with MR defecography relate to how the test is ordered. If only dynamic MRI of the pelvis is ordered, an evacuation phase may not be performed. It is imperative that an evacuation phase be undertaken; otherwise, significant underestimation of the perineal descent and other potential abnormalities can occur [44]. Similarly, the nature of the rectal contrast can influence measurements, with evidence suggesting that ultrasound gel might underestimate rectal abnormalities and perineal descent measurements compared with a potato starch mixture, although the difference in descent measurements was not significant [45].
We feel that both contemporary defecography and MR defecography are essentially equivalent in the diagnosis of DPS and either test is a reasonable choice in the workup of a patient. If MR defecography is used, it is important for the provider to be aware of potential limitations to interpret the results properly.
Physical examination can be helpful in observing perineal descent, but has significant limitations and must be coupled with symptom assessment to accurately diagnose the syndrome and assess for concurrent pathological conditions. Although the use of perineometers to measure perineal descent has been suggested, there is evidence that these devices significantly underestimate the amount of descent as they do not truly simulate defecation, measuring the anal verge rather than the anorectal angle, and being significantly affected by body fat over the ischial tuberosities, which alters the reference point [31]. Examination of the peri-anal region may show signs of posterior vaginal wall prolapse, rectal mucosa prolapse, and erythema/irritation [23]. Rectal examination often yields a feeling of bulging from the anterior rectal wall. Most significant is the caudal laxity of the perineum with digital examination traction and the rectal ampulla filling anteriorly and caudally with strain. Anal sphincter tone can also be normal or weakened.
Altered PNMTL values, which could suggest pudendal neuropathy, can be used as additional testing in equivocal cases although the lack of consistent findings on PNMTL makes this less useful [29, 30].
It is also important for the practicing provider to be aware of other potential etiologies and coexisting pathological conditions in patients with obstructed defecation. Rectocele, sigmoidocele, enterocele, anismus, solitary rectal ulcer syndrome, intussusception, and malignancy are only a few of the many other causes that one must keep in mind during a workup for obstructed defecation [46–48].
Treatment
Initial management of DPS generally consists of conservative measures in the form of laxatives, suppository use, enema use, and biofeedback [15, 46, 49]. Some advocate the use of a high-fiber diet, although there is concern that this may only exacerbate the problem at hand [15]. The utility of biofeedback in patients with DPS is varied in the literature, with success rates ranging from 30 to 50 % [9, 50]. It also appears that women with a smaller degree of perineal descent responded more favorably to biofeedback [9]. Limitations of biofeedback for obstructed defecation, as with other pelvic floor conditions, are related to the short duration of effectiveness and the need for retraining, which can be cumbersome for patients [51, 52]. Although not FDA-approved, specialized commode seats with support arms are available and have been suggested as ways to potentially alleviate symptoms (Fig. 7) [50, 53].
Modified commode seat with support. An example of several devices on the market that potentially offer mechanical support to the perianal region. This should theoretically increase the anorectal angle and allow for defecation to be completed without significant straining and minimize the amount of perineal descent. (Used with permission by Mecha-Medic Solution, Pulau Pinang, Malaysia)
It is controversial whether surgical management is even an option for patients with DPS. Cundiff et al. described a modification of the traditional abdominal sacrocolpopexy into a colpoperineopexy coupled with Halban’s culdoplasty, with promising short-term results in women with various stages of pelvic organ prolapse, though not specifically addressing women with DPS. The study was limited by a small sample size, inconsistent diagnosis of DPS, and short-term follow-up [54]. This technique was further discussed using a laparoscopic approach in a case report, which again limits the application of the procedure to a larger population [55]. Retrospective data suggest a correlation between women with DPS who underwent an abdominal hysterectomy and the subsequent development of fecal incontinence [14]. Similarly, there is evidence that women with increased perineal descent have higher rates of previous hysterectomy [56]. Although no causation is shown, this further illustrates the challenges in addressing this syndrome with a surgical procedure.
A case series on a transperineal approach consisting of levator plate myorrhaphy was shown to improve symptoms and decrease perineal descent. Because of the small sample size of 9 patients, the lack of defecography for diagnosis and short-term follow-up, this technique has limited application [57]. One of the only randomized control trials available looked at the efficacy of two transanal staple approaches and found a significant reduction in perineal descent radiographically and an improvement in constipation symptoms [58]. Others feel that surgical management plays no role other than to potentially offer a diverting stoma for patients in severe cases [12]. At this point, there is certainly no consensus on a surgical procedure that can be recommended as being ideal for the treatment of DPS. We have attempted to create a diagnostic and treatment algorithm that may be useful to the practicing clinician (Fig. 8).
Conclusion
Descending perineal syndrome is a phenomenon that is difficult to treat and can cause obstructed defecation. The relatively sparse prospective research on DPS limits the conclusions that can be drawn in this narrative review. Overall, DPS can lead to significant quality of life disturbances for patients. Accurate recognition based on patient symptoms, physical examination, and imaging are fundamental in making a correct diagnosis followed by initiation of conservative therapy to alleviate symptoms and allow for patients to better manage their bowel function. In very select cases, surgical therapy may offer some benefit, but until more prospective trials are performed to evaluate long-term efficacy and appropriate patient selection, surgical management has limited application.
References
Porter NH (1962) A physiological study of the pelvic floor in rectal prolapse. Ann R Coll Surg Engl 31:379–404
Parks AG, Porter NH, Hardcastle J (1966) The syndrome of the descending perineum. Proc R Soc Med 59:477–482
Hardcastle JD (1969) The descending perineum syndrome. Practitioner 203:612–619
Shorvon PJ, McHugh S, Diamant NE, Somers S, Stevenson GW (1989) Defecography in normal volunteers: results and implications. Gut 30:1737–1749
Mahieu P, Pringot J, Bodart P (1984) Defecography. I. Description of a new procedure and results in normal patients. Gastrointest Radiol 9:247–251
Ekberg O, Nylander G, Fork FT (1985) Defecography. Radiology 155:45–48
Bartram CI, Turnbull GK, Lennard-Jones JE (1988) Evacuation proctography: an investigation of rectal expulsion in 20 subjects without defecatory disturbance. Gastrointest Radiol 13:72–80
Faccioli N, Comai A, Mainardi P, Perandini S, Moore F, Pozzi-Mucelli R (2010) Defecography: a practical approach. Diagn Interv Radiol 16:209–216
Harewood GC, Coulie B, Camilleri M, Rath-Harvey D, Pemberton JH (1999) Descending perineum syndrome: audit of clinical and laboratory features and outcome of pelvic floor retraining. Am J Gastroenterol 94:126–130
Choi JS, Wexner SD, Nam YS, Mavrantonis C, Salum MR, Yamaguchi T, Weiss EG, Nogueras JJ, Yu CF (2000) Intraobserver and interobserver measurements of the anorectal angle and perineal descent in defecography. Dis Colon Rectum 43:1121–1126
Jorge JM, Ger GC, Gonzalez L, Wexner SD (1994) Patient position during cinedefecography. Influence on perineal descent and other measurements. Dis Colon Rectum 37:927–931
Landmann RG, Wexner SD (2008) Paradoxical puborectalis contraction and increased perineal descent. Clin Colon Rectal Surg 21:138–145
Armañanzas L, Arroyo A, Ruiz-Tovar J, López A, Santos J, Moya P, Gómez MA, Candela F, Calpena R (2015) Chronic idiopathic anal pain. Results of a diagnostic-therapeutic protocol in a colorectal referral unit. Cir Esp 93:34–38
Pucciani F, Boni D, Perna F, Bassotti G, Bellini M (2005) Descending perineum syndrome: are abdominal hysterectomy and bowel habits linked? Dis Colon Rectum 48:2094–2099
Pemberton JH (1990) Anorectal and pelvic floor disorders: putting physiology into practice. J Gastroenterol Hepatol 5:127–143
Henry MM, Parks AG, Swash M (1982) The pelvic floor musculature in the descending perineum syndrome. Br J Surg 69:470–472
Bartolo DC, Read NW, Jarratt JA, Read MG, Donnelly TC, Johnson AG (1983) Differences in anal sphincter function and clinical presentation in patients with pelvic floor descent. Gastroenterology 85:68–75
Read NW, Bartolo DC, Read MG, Hall J, Haynes WG, Johnson AG (1983) Differences in anorectal manometry between patients with haemorrhoids and patients with descending perineum syndrome: implications for management. Br J Surg 70:656–659
Womack NR, Morrison JF, Williams NS (1986) The role of pelvic floor denervation in the aetiology of idiopathic faecal incontinence. Br J Surg 73:404–407
Palit S, Lunniss PJ, Scott SM (2012) The physiology of human defecation. Dig Dis Sci 57:1445–1464
Ambrose S, Keighley MR (1985) Outpatient measurement of perineal descent. Ann R Coll Surg Engl 67:306–308
Chang J, Chung SS (2012) An analysis of factors associated with increased perineal descent in women. J Korean Soc Coloproctol 28:195–200
Baek HN, Hwang YH, Jung YH (2010) Clinical significance of perineal descent in pelvic outlet obstruction diagnosed by using defecography. J Korean Soc Coloproctol 26:395–401
Sunderland S (1978) Nerves and nerve injuries. Churchill Livingstone, New York, pp 62–66
Henry MM, Parks AG, Swash M (1980) The anal reflex in idiopathic faecal incontinence: an electrophysiological study. Br J Surg 67:781–783
Wall EJ, Massie JB, Kwan MK, Rydevik BL, Myers RR, Garfin SR (1992) Experimental stretch neuropathy. Changes in nerve conduction under tension. J Bone Joint Surg (Br) 74:126–129
Ho YH, Goh HS (1995) The neurophysiological significance of perineal descent. Int J Colorectal Dis 10:107–111
Kiff ES, Barnes PR, Swash M (1984) Evidence of pudendal neuropathy in patients with perineal descent and chronic straining at stool. Gut 25:1279–1282
Jorge JM, Wexner SD, Ehrenpreis ED, Nogueras JJ, Jagelman DG (1993) Does perineal descent correlate with pudendal neuropathy? Dis Colon Rectum 36:475–483
Vaccaro CA, Wexner SD, Teoh TA, Choi SK, Cheong DM, Salanga VD (1995) Pudendal neuropathy is not related to physiologic pelvic outlet obstruction. Dis Colon Rectum 38:630–634
Oettle GJ, Roe AM, Bartolo DC, Mortensen NJ (1985) What is the best way of measuring perineal descent? A comparison of radiographic and clinical methods. Br J Surg 72:999–1001
Mellgren A, Bremmer S, Johansson C, Dolk A, Udén R, Ahlbäck SO, Holmström B (1994) Defecography. Results of investigations in 2,816 patients. Dis Colon Rectum 37:1133–1141
Varma MG, Hart SL, Brown JS, Creasman JM, Van Den Eeden SK, Thom DH (2008) Obstructive defecation in middle-aged women. Dig Dis Sci 53:2702–2709
Yang A, Mostwin JL, Rosenshein NB, Zerhouni EA (1991) Pelvic floor descent in women: dynamic evaluation with fast MR imaging and cinematic display. Radiology 179:25–33
Lamb GM, de Jode MG, Gould SW, Spouse E, Birnie K, Darzi A, Gedroyc WM (2000) Upright dynamic MR defaecating proctography in an open configuration MR system. Br J Radiol 73:152–155
Mortele KJ, Fairhurst J (2007) Dynamic MR defecography of the posterior compartment: indications, techniques and MRI features. Eur J Radiol 61:462–472
Goei R, Kemerink G (1990) Radiation dose in defecography. Radiology 176:137–139
Bertschinger KM, Hetzer FH, Roos JE, Treiber K, Marincek B, Hilfiker PR (2002) Dynamic MR imaging of the pelvic floor performed with patient sitting in an open-magnet unit versus with patient supine in a closed-magnet unit. Radiology 223:501–508
Foti PV, Farina R, Riva G, Coronella M, Fisichella E, Palmucci S, Racalbuto A, Politi G, Ettorre GC (2013) Pelvic floor imaging: comparison between magnetic resonance imaging and conventional defecography in studying outlet obstruction syndrome. Radiol Med 118:23–39
Vanbeckevoort D, Van Hoe L, Oyen R, Ponette E, De Ridder D, Deprest J (1999) Pelvic floor descent in females: comparative study of colpocystodefecography and dynamic fast MR imaging. J Magn Reson Imaging 9:373–377
Kelvin FM, Maglinte DD, Hale DS, Benson JT (2000) Female pelvic organ prolapse: a comparison of triphasic dynamic MR imaging and triphasic fluoroscopic cystocolpoproctography. AJR Am J Roentgenol 174:81–88
Healy JC, Halligan S, Reznek RH, Watson S, Bartram CI, Phillips R, Armstrong P (1997) Dynamic MR imaging compared with evacuation proctography when evaluating anorectal configuration and pelvic floor movement. Am J Roentgenol 169:775–779
Schoenenberger AW, Debatin JF, Guldenschuh I, Hany TF, Steiner P, Krestin GP (1998) Dynamic MR defecography with a superconducting, open-configuration MR system. Radiology 206:641–646
Flusberg M, Sahni VA, Erturk SM, Mortele KJ (2011) Dynamic MR defecography: assessment of the usefulness of the defecation phase. Am J Roentgenol 196:394–399
Solopova AE, Hetzer FH, Marincek B, Weishaupt D (2008) MR defecography: prospective comparison of two rectal enema compositions. Am J Roentgenol 190:118–124
Andromanakos N, Skandalakis P, Troupis T, Filippou D (2006) Constipation of anorectal outlet obstruction: pathophysiology, evaluation and management. J Gastroenterol Hepatol 21:638–646
El-Nashar SA, Occhino JA, Trabuco E, Gebhart J, Klingele C (2014) Descending perineum syndrome: a fresh look at an interesting and complex pelvic floor disorder. J Minim Invasive Gynecol 21:S16–S17
Bartolo DC, Roe AM, Virjee J, Mortensen NJ, Locke-Edmunds JC (1988) An analysis of rectal morphology in obstructed defaecation. Int J Colorectal Dis 3:17–22
Khaikin M, Wexner SD (2006) Treatment strategies in obstructed defecation and fecal incontinence. World J Gastroenterol 12:3168–3173
Schey R, Cromwell J, Rao SS (2012) Medical and surgical management of pelvic floor disorders affecting defecation. Am J Gastroenterol 107:1624–1633
Guillemot F, Bouche B, Gower-Rousseau C, Chartier M, Wolschies E, Lamblin MD, Harbonnier E, Cortot A (1995) Biofeedback for the treatment of fecal incontinence. Long-term clinical results. Dis Colon Rectum 38:393–397
Dailianas A, Skandalis N, Rimikis MN, Koutsomanis D, Kardasi M, Archimandritis A (2000) Pelvic floor study in patients with obstructive defecation: influence of biofeedback. J Clin Gastroenterol 30:176–180
Beco J, Antolak S (2010) Posterior perineal support during defecation, descending perineum syndrome, pudendal neuropathy and anal fissures. Tech Coloproctol 14:193–194
Cundiff GW, Harris RL, Coates K, Low VH, Bump RC, Addison WA (1997) Abdominal sacral colpoperineopexy: a new approach for correction of posterior compartment defects and perineal descent associated with vaginal vault prolapse. Am J Obstet Gynecol 177:1345–1353
Link RE, Su LM, Bhayani SB, Wright EJ (2004) Laparoscopic sacral colpoperineopexy for treatment of perineal body descent and vaginal vault prolapse. Urology 64:145–147
Alves-Ferreira PC, Gurland B, Zutshi M, Hull T (2012) Perineal descent does not imply a more severe clinical disorder. Colorectal Dis 14:1372–1379
Beco J (2008) Interest of retro-anal levator plate myorrhaphy in selected cases of descending perineum syndrome with positive anti-sagging test. BMC Surg 8:1–13
Boccasanta P, Venturi M, Salamina G, Cesana BM, Bernasconi F, Roviaro G (2004) New trends in the surgical treatment of outlet obstruction: clinical and functional results of two novel transanal stapled techniques from a randomised controlled trial. Int J Colorectal Dis 19:359–369
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Chaudhry, Z., Tarnay, C. Descending perineum syndrome: a review of the presentation, diagnosis, and management. Int Urogynecol J 27, 1149–1156 (2016). https://doi.org/10.1007/s00192-015-2889-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-015-2889-0