Abstract
Introduction and hypothesis
Stress urinary incontinence (SUI) is managed with pelvic floor muscle training (PFMT), but the mechanism of treatment action is unclear. Resting maximal urethral closure pressure (MUCP) is lower in women with SUI, but it is unknown whether PFMT can alter resting MUCP. This systematic review evaluated whether voluntary pelvic floor muscle (PFM) contraction increases MUCP above its resting value (augmented MUCP) and the effect of PFMT on resting and augmented MUCP.
Methods
Experimental and effect studies were identified using PubMed and PEDro. The PEDro scale was used to assess internal validity of interventional studies.
Results
We identified 21 studies investigating the influence of voluntary PFM contraction in women. Comparison was hindered by varying demographics, antecedent history, reporting of confirmed correct PFM contraction, and urethral pressure profilometry (UPP) techniques. Mean incremental increase in MUCP during PFM contraction in healthy women was 8–47.3 cm H2O; in women with urinary incontinence (UI), it was 6–24 cm H2O. Nine trials reporting MUCP as an outcome of PFMT were found. Wide variation in PFMT regimes affected the findings. Two studies found significant improvement in MUCP of 5–18 cm H20. Seven studies assessed augmentation of MUCP with PFM contraction; mean increase was −0.1 to 25 cm H20.
Conclusions
There is no definitive evidence that PFMT increases resting MUCP as its mechanism of action in managing SUI. The degree to which a voluntary PFM contraction augments MUCP varies widely. There was evidence to suggest PFMT increases augmented MUCP. Drawing firm conclusions was hampered by study methodologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Level 1 evidence indicates that pelvic floor muscle training (PFMT) is effective in the treatment of female stress urinary incontinence (SUI). Accordingly, PFMT has grade A recommendation from expert consensus bodies, such as the International Consultations on Incontinence (ICI) and the European Association of Urology (EAU), as the first-line treatment for SUI and mixed urinary incontinence (MUI) [1–3]. Despite consensus regarding the efficacy of PFMT in both systematic reviews and meta-analyses, it is less clear how this treatment actually works [4]. A number of theories endeavor to explain its efficacy. First, altered PFM morphology causes a significant lift of both the bladder and rectal ampulla, reduction in the levator hiatus area, increased muscle thickness, and reduced muscle length. This was demonstrated in an assessor-blinded randomized controlled trial (RCT) following 6 months of PFMT [5]. Second, PFMT prevents descent of the bladder and the urethra during activities that cause increased intra-abdominal pressure (IAP), such as running, jumping, and coughing [4]. Third, it is proposed that PFMT increases the strength of a voluntary precontraction of the pelvic floor muscles (PFM) before increases in IAP (known as the Knack maneuver). A single-blinded randomized controlled trial (RCT) showed that teaching women the Knack, even without strength training, reduced urinary leakage by an average of 98.2 % during a medium cough test and by 73.3 % during a deep cough test [6]. Investigating PFM by electromyography (EMG) and perineal ultrasound revealed that the Knack significantly reduced downward movement of the bladder neck, implying increased structural support during a single PFM contraction [7]. Finally, PFMT facilitates an automatic, unconscious contraction of the PFM, increasing the maximal urethral closure pressure (MUCP) both at rest and during increases in IAP [4]. Recently, a case–control study found that resting MUCP was the one factor that differed most significantly between women with and without SUI, being 43 % lower in women with SUI (40.8 cm H2O ±17.1 versus 70.2 cm H2O ± 22.4 in SUI and continent women, respectively) [8]. Furthermore, analysis of data from >9000 women found that resting MUCP was significantly lower in those with SUI compared with continent women (48.2 cm H2O [95 % confidence intereval (CI) 47–49] versus 67 cm H2O (95 % CI 65–85, p < 0.001) [9]. Thus, insight into the influence of both a voluntary contraction and PFMT on MUCP is required.
At present, there is scant understanding of the minimum PFM strength required effectively to prevent leakage during the Knack. There has been little focus on investigating whether there is an association between PFM strength and MUCP. Furthermore, it is not known whether PFMT influences MUCP either at rest or during increases in IAP [10, 11]. Investigating these factors using urethral pressure profilometry (UPP) requires proper patient training and methodological standardization [12], since studies have shown high variation in normal values between different centers [13]. The Fifth International Consultation on Incontinence Committee on Urodynamic testing [10] noted variation of ± 10–15 % on test–retest data for a number of different parameters (including volume, pressure, and flow) and recommended that investigators and clinicians take into account the inherent physiological variation of UPP [10]. Evaluating the effect of a PFM contraction during MUCP is difficult, in part because many women perform a PFM contraction incorrectly [14]. This systematic literature review had two aims: (1) to determine the incremental increase in MUCP elicited by a single PFM contraction (augmented MUCP); (2) to evaluate the effect of PFMT on both resting and augmented MUCP.
Materials and methods
A search using PubMed was undertaken using the following search terms: pelvic floor muscles AND female AND urethra. This search was limited to the English language. Inclusion criteria were experimental studies, women, and MUCP increment during a single PFM contraction.
In addition, an advanced search on PEDro was done using the terms incontinence AND clinical trial. Clinical trials including pre- and posttest, randomized, and quasi-controlled trials designed to investigate the effect of PFMT on UPP variables were included. PFMT was defined as sets of PFM exercises over time, including use of biofeedback or weighted vaginal cones. Exclusion criteria were studies in animals, men, children, neurological diseases, pregnancy, and being postpartum. Clinical trials using electrical stimulation, magnetic chair, tibial nerve stimulation, and treatments other than PFMT were also excluded. In addition, we hand searched reference lists of retrieved papers and the reference list in the chapter “Urodynamic testing” of the ICI [10].
Internal validity of clinical trials was rated according to the PEDro scale [15], a 10-point scale giving one point for each of the following criteria for internal validity of intervention studies: random allocation, concealed allocation, baseline comparability, blinding of subjects, blinding of therapist, blinding of assessor, adequate follow-up (≥85 %), intention to treat (ITT), between-groups comparison, point estimates, and variability. The scale contains one additional criterion for external validity: eligibility. Eligibility is not used in scoring methodological quality and risk of bias. Hence, the top score is 10 for internal validity. PEDro is a reliable, valid, and comprehensive method for assessing methodological quality [15].
Two researchers independently assessed and classified the studies. Each study was classified according to preset criteria and are reported in two separate tables: one for experimental studies and one for clinical trials (Tables 1 and 2, respectively). Experimental studies were classified and tabled according to author/year, participants, UPP assessment methods, PFM contraction assessment, subject position, and MUCP findings. Clinical trials were classified and tabled according to author/year, participants, design/PEDro score, intervention, dropout/adherence, urodynamic assessment methods, urodynamic outcome, other outcomes, and comments. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews was followed [16].
Results
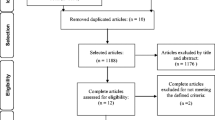
Selection of articles for inclusion in this systematic review is given in Fig. 1.
Experimental studies
Twenty-one studies investigating augmented MUCP were found (Table 1) [14, 17–36]. One study was excluded because the results combined values for men and women [37]. Studies were published between 1982 and 2012. The number of participants ranged from 11 [18] to 300 [14], and participants’ ages ranged from 18 to 90 years. In several studies it was not clear as to whether the investigators assessed the ability of patients to perform a PFM contraction correctly, either before or during urodynamic studies [14, 17, 18, 20–22, 25, 26, 30, 33].
Urodynamic methodology differed between studies. Prior to 2002, there was a lack of consensus regarding both a definition of the concept of urethral pressure and standardization of the measurement methodology [12, 38]. Two studies assessed UPP with the patient in the standing position [17, 23], but the majority was performed supine. In six studies, the bladder was empty [18, 21, 22, 31, 34, 36]. In contrast, Amaro et al. [28] obtained their findings with the bladder full. Three studies failed to state bladder volume [24, 27, 33].
Augmentation of MUCP by pelvic floor contraction
Mean incremental increase in MUCP during PFM contraction in healthy women ranged between 8 [18] and 47.3 cm H2O [27]. Incremental range in women with UI varied from 6 to 23.5 cm H20 [27, 34, 36]. Elser et al. [26] found increase in MUCP during a PFM contraction. Three studies compared women with SUI with continent women [21, 29, 33]. Across the studies, continent women had a higher augmented MUCP by 7-8cm H20; however, only two studies found a statistically significant difference (p < 0.01, p value not reported) [21, 33]. Lose [21] investigated women with SUI only; the other study compared women with a range of etiologies with age-matched controls.
One study defined whether their patients achieved an effective PFM contraction, judged as an arbitrarily defined increase to at least 120 % of resting MUCP [14]. When this criterion was applied to women with either SUI or prolapse, between 39 % and 60 % achieved an effective contraction. Furthermore, a number of studies reported a reduction in MUCP during PFM contraction compared with the resting measurement [14, 26, 32, 36]. All of these studies included women with possible injuries to the pelvic floor. Miller et al. [30] found a significantly lower increase in MUCP in those with complete absence of pubococcygeal muscle [assessed by magnetic resonance imaging (MRI)]; Brincat et al. [35] found no significant differences between women with and without major defects. Implicitly, inability to contract the pelvic floor voluntarily would preclude changes in MUCP with attempted pelvic floor contraction. However, alterations in MUCP may be detectable in some women unable to contract their pelvic floor. This observation may reflect an effect on MUCP of associated dynamics, such as downward pressure on the pelvic floor caused by abdominal straining. Two studies excluded women who were judged as being unable to perform an effective PFM contraction during vaginal palpation [23, 24]. In these studies, mean incremental increase in MUCP during a PFM contraction ranged from 9 to 23 cm H20, which is comparable with the overall range across all studies of 8 cm H2O [18] to 47.3 cm H2O [27]. Mayer et al. [23], in their study of >200 women, found that age negatively correlated with both MUCP and augmented MUCP. Several studies investigated the increase in closure pressure at different points along the urethra and were unable to ascertain where along the urethra the greatest increment in closure pressure occurs during a PFM contraction [17, 18, 21, 22].
PFMT clinical trials
Nine clinical trials reporting UPP as an outcome of PFMT were published between 1987 and 2008 (Table 2) [19, 20, 26, 29, 34, 39–42]. Three were RCTs [26, 39, 40], one was a nonrandomized controlled trial [20], and five were pre-and posttest designs [19, 29, 34, 41, 42]. The number of participants ranged from 26 to 204 [19, 26] and participant age ranged from 19 to 79 years [20]. The majority of trials investigated patients with urodynamic SUI [19, 20, 34, 39, 40, 42]. The remaining studies assessed patients with a range of diagnoses [26, 29, 41]. Six studies stated that PFM contraction was confirmed by vaginal palpation [19, 29, 34, 39, 40, 42]; the remaining studies did not report whether this was performed [20, 26, 41]. PEDro scores for controlled trials ranged between 2 and 8, with the three RCTs scoring ≥5. Only one trial published after 2002, by Teleman et al. [29], stated they adhered to International recommendations [12, 38].
There was wide variation in the PFMT regimes across the nine clinical studies. The time given to PFMT ranged from 6 weeks to 12 months [20, 34]. Five of the nine studies assessed the effect of PFMT after 3–4 months [19, 26, 29, 41, 42]. The amount of supervision provided by a physiotherapist or nurse varied from twice weekly [42] to one assessment followed by home exercises only [29]. The protocol for home exercises varied from 5–10 contractions every 30 min daily [20], to 8–12 repetitions three times per day [39, 40]. Two studies did not state whether participants were encouraged to perform home exercises [26, 42].
Effect of PFMT on resting MUCP
Only Benvenuti et al. [19] and Bø et al. [39] found a statistically significant improvement in MUCP following PFMT. The increase in MUCP in Bø et al.’s [39] study was 4.6 cm H2O in the intensive PFMT group compared with no significant increase in the home exercise group (p < 0.02). Benvenuti et al. [19] reported a greater increase—of 18cm H20—but there was no control group.
Effect of PFMT on augmented MUCP
Seven studies assessed MUCP with a PFM contraction [19, 20, 26, 29, 34, 40, 41]. Mean increase in augmented MUCP following PFMT varied from −0.1 cm H20 [26] to 25 cm H20 [19].
Effect of PFMT on other UPP measures
Of the five pre- and posttest studies that assessed functional urethral profile length (FUPL), only Benvenuti et al. [19] found a significant improvement. Two RCTs measured FUPL and neither found a significant change [26, 39]. Three studies, including two RCTs [26, 39], measured cough-pressure transmission ratio (PTR) as an outcome of PFMT. There were contradictory findings in the two RCTs; the study that found a significant difference only investigated women with SUI [39], while the other included women with a range of diagnoses [26]. Both studies used the microtip transducer technique for measuring UPP. One RCT investigating women with urodynamic SUI found that significantly fewer women had negative pressure in the intensive PFMT group (p < 0.01) [39]. In the pre- posttest study by Zahariou et al. [34], which included women with SUI, cough PTR significantly improved from 85 to 108 %, but this study had no control group.
Discussion
We conducted a systematic review to assess both the influence of a voluntary PFM contraction on MUCP and the effect of PFMT on MUCP and augmented MUCP. The wide inclusion criteria ensured a comprehensive systematic analysis of both a single PFM contraction and PFMT on MUCP across healthy women and those with either SUI or MUI. This allowed us to explore UPP findings within each of these categories of participants. Pooled analysis of UPP data suitable for meta-analysis was not possible due to the broad heterogeneity of urodynamic methods and urodynamic diagnoses. In particular, methods of UPP differed substantially between centers, which made direct comparsion invalid. Moreover, there was wide variation in PFMT protocols throughout the clinical trials, and this likewise made it invalid to pool results for meta-analysis.
Experimental studies
Our results indicate that there was a wide variance in augmented MUCP—from 8 to 33cm H20—in healthy women. Women with UI appear to produce a statistically significant smaller increment in augmented MUCP (by 7–8cm H20) than healthy women. It was not possible to make a meaningful prediction regarding the minimum augmented MUCP required to maintain continence for two reasons: the number of studies that compared healthy women with incontinent women were small, and methodologies used for UPP were varied, precluding pooling of analyses. MUCP value is dependent upon the type of catheter used, its orientation in the urethra, the quantity of fluid in the bladder, and patient examination position [12]. Studies evaluated in this review varied across all these parameters.
There is inherent variation in MUCP measurement of between 10 and 15 %. Indeed, both fluid perfusion and microtip urodynamic techniques may have low reliability [10]. Resting UPP measurement (including MUCP) using the fluid-perfusion technique produces a standard deviation (SD) that ranges from 3.3 to 8.1 cm H2O, with an approximate average of 5 cm H2O (95 % confidence limits ± 10 cm H2O), or ± 5 %. Using the microtip transducer technique produces an even greater range in SD 3.3–16.5 cm H2O. This means that the 95 % CI can be as large as ± 33 cm H2O. Furthermore, the coefficient of variance with this technique is 17 % (95 % confidence limits ± 34 %) [10]. Despite the inherent difficulties with study reliability, women with SUI are consistently found to have a lower MUCP [9].
The influence of age on MUCP has been documented [9, 43]. Despite this, only four studies had an age range of <10 years [18, 30, 34, 36]; the largest range was 69 years [36]. One study investigated the relationship between augmented MUCP and age and found a negative correlation [33], similar to other findings on resting MUCP [44–46]. Clearly, influence of age on augmented MUCP needs to be evaluated.
When attempting to determine the effect a PFM contraction has on MUCP, it is vital to confirm the presence of a correct PFM contraction, since significant numbers of women will not perform a PFM contraction correctly despite good verbal teaching [14]. Indeed, a number of studies found that MUCP reduced with a PFM contraction. Without evaluating whether women are performing a PFM contraction correctly, it is difficult to determine whether this reduction is caused simply by a Valsalva causing shift in the relative position of the recording catheter, or whether other mechanisms are at play. Regardless, nearly half of the studies reviewed did not report whether this was carried out.
It remains unclear whether significant injury to the PFM affects MUCP. A small study showed women with complete absence of pubococcygeus had a significantly lower increase in pressure than those with a fully intact muscle. However, when a defect was present, rather than complete absence, augmented MUCP was not significantly affected [35].
Clinical studies
While there was evidence to suggest that PFMT increased augmented MUCP—with the increment ranging from 4 to 25 cm H20—the various studies showed wide variation in the extent of effect on MUCP and the reporting of comparative information for the extent of change in MUCP parameters for subgroups (most notably, whether SUI symptoms resolved or otherwise). Thus, we are unable to state that published evidence supports these mechanisms of action as the basis of successful treatment of SUI by PFMT. It remains conceivable that increased MUCP is a factor in PFMT response, but future research assessing this idea must have a robust experimental design standardizing urodynamic methodology, PFM assessment, compliance with PFMT, and outcome reporting.
It was not possible to conclude whether a minimum increase is required to achieve continence, because subgroup analysis comparing successful with unsuccessful outcome was not available.
It was also difficult to draw firm conclusions, because key aspects that ensure that PFMT was effective were not used consistently: confirmation of a correct contraction, adherence, training duration, and dosage [11]. Slightly fewer than half the studies did not report whether vaginal palpation was performed to confirm a correct contraction. PFMT can only be effective when it is actually performed. Previous research has demonstrated that adherence can vary widely [11]. Only two studies reported adherence rates; indeed, no study investigating augmented MUCP reported this data. Adaptation to strength training appears to proceed in a linear way during the first 6 months of training [47], and only three studies were carried out over this period [34, 39, 40]. Just two studies used muscle training regimes according to recommendations by the American College of Sports Medicine: three sets daily of 8-12 repetitions [48].
There is a lack of consensus regarding UPP as a diagnostic test, and so it is mainly used in specialized centers as an adjunctive technique in clinical urodynamic assessment. However, for the purposes of this review, we assessed whether UPP can help explain PFMT mechanism of action, regardless of the limited clinical use of UPP. The clinical trials in this review often included only scant data about UPP methodology, making it impossible to ascertain whether similar urodynamic methodology was used. There is no agreed-upon approach to ensure high quality (reliable and valid) urodynamic testing during PFM contraction and MUCP measurement before and after PFMT. The ICS published standards on urethral pressure measurement in 2002 [12, 38], but internal and external consistency, retest reliability, and sensitivity to change have never been quantified. Accordingly, centers adapt methodology according to various influences, and extrapolation of findings between centers is thus unreliable.
Conclusion
There is wide variation in the degree to which a PFM contraction augments MUCP. There is minimal evidence to support the theory that PFMT produces a significant increase in MUCP. However, both PFM strength [49] and UPP [12, 38] are difficult variables to measure with objective reliability. There are also problems with validity when evaluating whether women perform a PFM contraction correctly during the procedure, as this factor was not documented. Future investigations of the association between measured PFM strength and different forms of urethral pressures is required, with the use of reliable and valid methods, using standardized UPP measurements and correct instructions to patients.
References
NICE (2006) Urinary Incontinence: The management of urinary incontinence in women. National Institute for Health and Clinical Excellence Clinical Guideline 40: http://www.nice.org.uk/nicemedia/pdf/word/CG40quickrefguide1006.pdf
Abrams P, Cardozo L, Khoury S, Wein A (eds) (2013) Incontinence: 5th International Consultation on Incontinence. European Association of Urology/ International Consultation on Urological Diseases, Paris, France
Thuroff JW, Abrams P, Andersson KE et al (2011) EAU guidelines on urinary incontinence. Eur Urol 59:387–400
Bo K (2004) Pelvic floor muscle training is effective in treatment of female stress urinary incontinence, but how does it work? Int Urogynecol J Pelvic Floor Dysfunct 15:76–84
Braekken IH, Majida M, Engh ME, Bo K (2010) Morphological changes after pelvic floor muscle training measured by 3-dimensional ultrasonography: a randomized controlled trial. Obstet Gynecol 115:317–324
Miller JM, Ashton-Miller JA, DeLancey JO (1998) A pelvic muscle precontraction can reduce cough-related urine loss in selected women with mild SUI. J Am Geriatr Soc 46:870–874
Peschers UM, Fanger G, Schaer GN, Vodusek DB, DeLancey JO, Schuessler B (2001) Bladder neck mobility in continent nulliparous women. BJOG : Int J Obstet Gynaecol 108:320–324
Delancey JO (2010) Why do women have stress urinary incontinence? Neurourol Urodyn 29(Suppl 1):S13–S17
Kapoor D, White P, Housami F, Swithinbank L, Drake MJ (2012) Maximum urethral closure pressure in women: normative data and evaluation as a diagnostic test. Int Urogynecol J 23:1613–1618
Rosier P, Kuo H-C, De Gennaro M et al (2013) Urodynamic testing. In: Abrams PCL, Khoury S, Wein A (eds) Incontinence 5th International Consultation on Incontinence. European Association of Urology/ International Consutation on Urological Diseases, Paris
Hay-Smith EJ, Herderschee R, Dumoulin C, Herbison GP (2011) Comparisons of approaches to pelvic floor muscle training for urinary incontinence in women. The Cochrane database of systematic reviews: CD009508
Lose G, Griffiths D, Hosker G et al (2002) Standardisation of urethral pressure measurement: report from the Standardisation Sub-Committee of the International Continence Society. Neurourol Urodyn 21:258–260
Weber AM (2001) Is urethral pressure profilometry a useful diagnostic test for stress urinary incontinence? Obstet Gynecol Surv 56:720–735
Bump RC, Hurt WG, Fantl JA, Wyman JF (1991) Assessment of Kegel pelvic muscle exercise performance after brief verbal instruction. Am J Obstet Gynecol 165:322–327, discussion 7-9
Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M (2003) Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 83:713–721
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62:1006–1012
Constantinou CE, Govan DE (1982) Spatial distribution and timing of transmitted and reflexly generated urethral pressures in healthy women. J Urol 127:964–969
Colstrup H (1985) Voluntary contractions in the female urethra. J Urol 134:902–906
Benvenuti F, Caputo GM, Bandinelli S, Mayer F, Biagini C, Sommavilla A (1987) Reeducative treatment of female genuine stress incontinence. Am J Phys Med 66:155–168
Wilson PD, Al Samarrai T, Deakin M, Kolbe E, Brown AD (1987) An objective assessment of physiotherapy for female genuine stress incontinence. Br J Obstet Gynaecol 94:575–582
Lose G (1991) Urethral pressure and power generation during coughing and voluntary contraction of the pelvic floor in females with genuine stress incontinence. Br J Urol 67:580–585
Lose G, Colstrup H (1991) Urethral pressure and power generation during coughing and voluntary contraction of the pelvic floor in healthy females. Br J Urol 67:573–579
Mayer R, Wells TJ, Brink CA, Clark P (1994) Correlations between dynamic urethral profilometry and perivaginal pelvic muscle activity. Neurourol Urodyn 13:227–235
Bo K, Talseth T (1997) Change in urethral pressure during voluntary pelvic floor muscle contraction and vaginal electrical stimulation. Int Urogynecol J Pelvic Floor Dysfunct 8:3–6, discussion -7
van Loenen NT, Vierhout ME (1997) Augmentation of urethral pressure profile by voluntary pelvic floor contraction. Int Urogynecol J Pelvic Floor Dysfunct 8:284–287
Elser DM, Wyman JF, McClish DK, Robinson D, Fantl JA, Bump RC (1999) The effect of bladder training, pelvic floor muscle training, or combination training on urodynamic parameters in women with urinary incontinence. Continence Program for Women Research Group. Neurourol Urodyn 18:427–436
Kuo H (2000) The relationships of urethral and pelvic floor muscles and the urethral pressure measurements in women with stress urinary incontinence. Eur Urol 37:149–155
Amaro JL, Oliveira Gameiro MO, Padovani CR (2003) Treatment of urinary stress incontinence by intravaginal electrical stimulation and pelvic floor physiotherapy. Int Urogynecol J Pelvic Floor Dysfunct 14:204–208, discussion 8
Teleman PM, Gunnarsson M, Lidfeldt J, Nerbrand C, Samsioe G, Mattiasson A (2003) Urethral pressure changes in response to squeeze: a population-based study in healthy and incontinent 53- to 63-year-old women. Am J Obstet Gynecol 189:1100–1105
Miller JM, Umek WH, Delancey JO, Ashton-Miller JA (2004) Can women without visible pubococcygeal muscle in MR images still increase urethral closure pressures? Am J Obstet Gynecol 191:171–175
Baessler K, Miska K, Draths R, Schuessler B (2005) Effects of voluntary pelvic floor contraction and relaxation on the urethral closure pressure. Int Urogynecol J Pelvic Floor Dysfunct 16:187–190, discussion 90-1
Dannecker C, Hillemanns P, Strauss A, Hasbargen U, Hepp H, Anthuber C (2005) Episiotomy and perineal tears presumed to be imminent: the influence on the urethral pressure profile, analmanometric and other pelvic floor findings--follow-up study of a randomized controlled trial. Acta Obstet Gynecol Scand 84:65–71
Teleman PM, Mattiasson A (2007) Urethral pressure response patterns induced by squeeze in continent and incontinent women. Int Urogynecol J Pelvic Floor Dysfunct 18:1027–1031
Zahariou A, Karamouti M, Georgantzis D, Papaioannou P (2008) Are there any UPP changes in women with stress urinary incontinence after pelvic floor muscle exercises? Urol Int 80:270–274
Brincat CA, Delancey JO, Miller JM (2011) Urethral closure pressures among primiparous women with and without levator ani muscle defects. Int Urogynecol J 22:1491–1495
Dietz HP, Shek KL (2012) Levator function and voluntary augmentation of maximum urethral closure pressure. Int Urogynecol J 23:1035–1040
Shafik A, El-Sibai O (2001) Effect of levator ani muscle contraction on urethrovesical and anorectal pressures and role of the muscle in urination and defecation. Urology 58:193–197
Schafer W, Abrams P, Liao L et al (2002) Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn 21:261–274
Bo K, Talseth T, Holme I (1999) Single blind, randomised controlled trial of pelvic floor exercises, electrical stimulation, vaginal cones, and no treatment in management of genuine stress incontinence in women. BMJ 318:487–493
Kuo HC (2003) Videourodynamic results in stress urinary incontinence patients after pelvic floor muscle training. J Formos Med Assoc Taiwan yi zhi 102:23–29
Moreno AL, Benitez CM, Castro RA, Girao MJ, Baracat EC, de Lima GR (2004) Urodynamic alterations after pelvic floor exercises for treatment of stress urinary incontinence in women. Clin Exp Obstet Gynecol 31:194–196
Schick E, Tessier J, Bertrand PE, Dupont C, Jolivet-Tremblay M (2003) Observations on the function of the female urethra: I: relation between maximum urethral closure pressure at rest and urethral hypermobility. Neurourol Urodyn 22:643–647
Hendriksson L, Andersson K, Ulmsten U (1979) The urethral pressure profiles in continence and incontinent women. Scand J Urol Nephrol 13:5–10
Murphy M, Culligan PJ, Graham CA, Kubik KM, Heit MH (2004) Is the leak point pressure alone an accurate indicator of intrinsic sphincteric deficiency? Int Urogynecol J Pelvic Floor Dysfunct 15:294–297
Pfisterer MH, Griffiths DJ, Schaefer W, Resnick NM (2006) The effect of age on lower urinary tract function: a study in women. J Am Geriatr Soc 54:405–412
Folland JP, Williams AG (2007) The adaptations to strength training : morphological and neurological contributions to increased strength. Sports Med 37:145–168
Garber CE, Blissmer B, Deschenes MR et al (2011) American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43:1334–1359
Bo K, Sherburn M (2005) Evaluation of female pelvic-floor muscle function and strength. Phys Ther 85:269–282
Bo K, Hagen RH, Kvarstein B, Jørgensen J, Larsen S (1990) Pelvic floor muscle exercise for the treatment of female stress urinary incontinence: III. Effects of two different degrees of pelvic floor muscle exercise. Neurourol Urodyn 9:489–502
Acknowledgments
The senior author received a travelling fellowship from the International Continence Society. The first and second authors contributed equally to the preparation of this manuscript.
Funding
An ICS Fellowship awarded to Prof K. Bø funded a 4-week visit to Bristol Urological Institute to undertake this systematic review.
Conflicts of Interest
MJD is speaker and advisory board member for Allergan, Apogepha, Astellas, Ferring, and Pfizer. The other authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Maria Zubieta and Rebecca L. Carr contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zubieta, M., Carr, R.L., Drake, M.J. et al. Influence of voluntary pelvic floor muscle contraction and pelvic floor muscle training on urethral closure pressures: a systematic literature review. Int Urogynecol J 27, 687–696 (2016). https://doi.org/10.1007/s00192-015-2856-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-015-2856-9