Abstract
Introduction
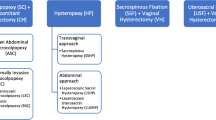
Pelvic organ prolapse is showing an increasing prevalence (3 – 50 %). The gold standard treatment of apical prolapse is sacrocolpopexy which can be performed via minimal access (laparoscopy or robotics) or open approaches. The aim of this review was to appraise the effectiveness of minimal access surgery versus the open approach in the treatment of apical prolapse.
Methods
Keywords were searched in: CINAHL, MEDLINE, CENTRAL, Cochrane MDSG Trials Register, Cochrane Library, Current Controlled Trials, ClinicalTrials.gov, WHO International Trials Registry Platform search portal, LILACS, and Google Scholar databases. Data up to 31 April 2014 were considered. Randomized and nonrandomized controlled trials evaluating all women who underwent minimally invasive sacropexy (MISC) and open sacropexy (OSC) were included. A data extraction tool was used for data collection. MISC was compared with OSC using narrative analysis and meta-analysis (RevMan) where appropriate.
Results
MISC and OSC were compared in 12 studies involving 4,757 participants. MISC and OSC were equally effective in terms of point-C POP-Q measurements and recurrence rate. MISC was associated with a lower transfusion rate (odds ratio 0.41, 95 % CI 0.20 – 0.83), shorter length of hospital stay (mean difference −1.57 days, 95 % CI −1.91 – −1.23 days), and less blood loss (mean difference −113.27 mL, 95 % CI −163.67 – −62.87 mL) but a longer operating time (mean difference 87.47, 95 % CI 58.60 – 116.34, p < 0.0001).
Conclusions
MISC showed similar anatomic results to OSC with a lower transfusion rate, shorter length of hospital stay and less blood loss. The rate of other complications was similar between the approaches. Cautious interpretation of results is advised due to risk of bias caused by the inclusion of nonrandomized studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pelvic organ prolapse has a prevalence of 3 – 6 % when based on symptoms and up to 50 % when based on vaginal examination [1]. Specifically, prevalence of apical prolapse ranges between 0.2 % and 43 % [2]. In the UK it represents approximately 1.1 % of the NHS (National Health Service) budget [3]. In England, 16.9 % of women were admitted in 2009 for treatment of pelvic organ prolapse, representing a cost of €81,030,907 [4].
Open sacrocolpopexy is considered the gold standard surgical treatment of apical prolapse [5]. However, it is associated with longer time to return to daily activities, longer operating times (OT) and greater costs [5]. Therefore, laparoscopic surgery has gained support amongst surgeons because of its advantages, but its marked learning curve has limited its adoption. Conversely, robotic surgery has arisen as the latest technology capable of offering benefits in terms of dexterity and shortening the learning curve [6].
The aim of this review was to appraise the effectiveness and safety of minimal access surgery in relation to the open approach in the treatment of apical prolapse to determine the best approach to performing sacropexy. Currently, there are three systematic reviews covering a similar topic. The first [7] included observational studies up to 2010, and the second was a Cochrane review [8]. From this date onwards, a variety of new research studies have been completed, which justifies an update on this topic. Additionally, just one and two studies, respectively, related to sacropexy were included in each review, and this may raise doubts concerning transferability [9]. Moreover, neither of these reviews compared minimally invasive sacropexy (MISC) and open sacropexy (OSC). The third systematic review [10] focused on robotic sacropexy (RSC) and did not include (among other studies) a recently published randomized controlled trial (RCT) comparing RSC and laparoscopic sacropexy (LSC). Additionally, this third review also included uncontrolled studies which are more susceptible to bias [11].
Objectives of the review
-
To compare the effectiveness of MISC and OSC in the treatment of apical prolapse.
-
To appraise the outcomes of MISC and OSC in relation to intraoperative and postoperative complications, mortality, postoperative length of stay (LOS), postoperative pain, estimated blood loss (EBL), OT and quality of life (QoL).
Research question
Which approach (MISC or OSC) is more effective when performing a sacropexy to treat women with prolapse of the apical segment of the vagina?
Methods
Selected databases were systematically searched using relevant key words (Table 1) to identify pertinent studies. The selected databases included: Cumulative Index to Nursing and Allied Health Literature (CINAHL), MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Menstrual Disorders and Subfertility Group (MDSG) Trials Register, Cochrane Library, Current Controlled Trials, ClinicalTrials.gov, WHO International Trials Registry Platform search portal, Latin American and Caribbean Health Science Literature (LILACS), and Google Scholar. Additionally, the reference list of relevant studies, the International Urogynecology Journal and the Journal of Minimally Invasive Gynecology [9] were also searched by hand.
Duplicate titles and abstracts were removed and the remaining studies were selected according to relevance and the use of a flow diagram. Abstracts were assessed against the inclusion and exclusion criteria and reasons for exclusion were documented. Two authors assessed selected full-text papers and those that did not meet the inclusion criteria were excluded. Finally, chosen papers were appraised rigorously to determine their quality using appropriate tools.
Criteria for study selection
Inclusion criteria
-
All women with prolapse of the apical segment of the vagina who underwent OSC or MISC (RSC or LSC)
-
Studies written in English, Portuguese, or Spanish
-
Studies from 1980 to 2014
-
Quantitative studies including experimental studies (RCTs), quasiexperimental studies (controlled before-and-after studies), and controlled observational studies (cohort studies)
-
Studies evaluating effectiveness of sacropexy, complications, LOS, EBL, OT, mortality, postoperative pain or postoperative QoL
Exclusion criteria
-
All women who underwent a different procedure for the surgical repair of apical prolapse
-
Studies not written in English, Portuguese, or Spanish
-
Case control studies, uncontrolled before-and-after studies, cross-sectional surveys, case series
-
Studies in cadavers
Quality assessment
The criteria to determine whether the included studies had low, high or unclear risk of bias were based on the appraisal tool recommended by the Cochrane Collaboration [9], which explores selection bias, performance bias, detection bias, attrition bias and reporting bias. Other biases were also considered: whether funding and conflicts of interests were reported in the included studies [9]. The Newcastle-Ottawa scale (NOS) was also used for quality assessment of nonrandomized studies because it provides an objective approach to the evaluation of validity that can be fully reported [9]. It evaluates eight items and each one can be awarded one star, except the item “comparability” that can be awarded two stars (maximum of nine). The NOS was customized to this research choosing two relevant confounders. “Previous abdominal surgery” was selected because it is an independent predictor of potential complications after surgery [12] whereas “concomitant surgery” is a variable related to longer OT [13]. Also, it was decided that the period of follow-up should be no less than 12 months to allow time to detect prolapse recurrence [14]. The maximum acceptable percentage of subjects lost to follow-up was agreed to be 20 % [15].
Data extraction
A data extraction tool was developed based on the aim, objectives and research question of this review. Effectiveness was defined as point-C POP-Q measurement at more than 1 cm above the level of the hymen [16]. Subjective effectiveness was defined as the woman reporting no symptoms after the procedure [16]. Other outcomes of interest were: LOS, EBL, OT and postoperative QoL. Complications and mortality were also recorded to assess the safety of the procedure. Authors were contacted by email to acquire missing information but no responses were obtained.
Data analysis
Narrative analysis and meta-analysis were used. For studies with different designs, narrative analysis was implemented using tables [17]. Review Manager 5.3 was used for meta-analysis [17]. For categorical outcomes, the odds ratio (OR) was calculated using the Mantel-Haenszel method [9]. For continuous variables, the mean difference (MD) was derived from means and standard deviations and used when outcomes were reported using identical scales. When scales were different, the standardized MD (SMD) was derived using RevMan [9]. The confidence interval was set at 95 % and p values <0.05 (two-tailed) were considered statistically significant. Papers in which authors reported outcomes using medians and ranges were not included in the meta-analysis [9].
RevMan was used to calculate heterogeneity using the chi-squared test and the percentage variability (I 2). When heterogeneity was lower than 50 %, fixed-effects meta-analysis was performed. When heterogeneity was equal to or higher than 50 %, heterogeneity was explored (i.e. for possible causes of heterogeneity) and, additionally, a random-effects meta-analysis was undertaken [9]. Subgroup analyses were performed where appropriate. A sensitivity analysis was also performed. This involved inclusion of high-quality studies (i.e. including RCTs and excluding observational studies NOS scores of five or less), reconsideration of the methods used for analysis (appropriate usage of fixed-effects versus random-effects methods, or MD versus SMD) and making assumptions on how missing data could have affected the results (assigning lost subjects to the worst case scenario) [9, 11].
Results
Details of studies
Full reports of 28 studies were appraised. The process of screening and selection is illustrated using a PRISMA flow chart (Fig. 1). After assessment of 28 studies, eight were excluded and the reasons are summarized in Table 2.
Two comparisons were made: MISC (i.e. RSC plus LSC) versus OSC which is presented in this review, and RSC versus LSC which is discussed in another review. Papers were therefore divided accordingly. From 20 studies included in the analysis, 12 compared MISC and OSC (Table 3) and eight RSC and LSC. However, the study by Nosti et al. [12] also compared RSC and LSC, and thus was included in both analyses.
Characteristics of studies comparing MISC and OSC
The characteristics of the studies comparing MISC and OSC are summarized in Table 3.
Study design
Of 12 studies, one was a RCT, two were prospective cohort studies and nine were retrospective cohort studies with a total of 4,757 subjects (Table 3). Furthermore, five studies compared RSC and OSC, four compared LSC and OSC and three compared MISC (RSC plus LSC) and OSC.
Technique used
Overall, five ports were used for RSC [12, 18, 19], whereas four ports were used for LSC [8, 20, 21]. In nine studies a concomitant hysterectomy was performed [12, 18, 19, 21–26]. Seven studies [12, 20, 21, 23, 24, 26, 27] reported the involvement of experienced surgeons, but in three studies [19, 22, 28] some surgeons were in their learning curve.
Quality appraisal
Figure 2 summarizes the risk of bias. Cohort studies were further appraised using the NOS (Table 4). Three studies [20, 24, 28] scored five stars, seven [12, 19, 21–23, 25, 26] scored six stars and one [18] scored seven stars.
Selection bias
One study [27] randomized subjects appropriately but failed to use adequate methods of allocation concealment. Inclusion of nonrandomized studies resulted in a high risk of selection bias (Fig. 3). Nevertheless, almost all cohort studies had a NOS score of three stars in the category “selection” (Table 4).
Performance bias
Only in one study [27] was the ward staff who supervised analgesic requirements blinded. It was not possible to blind subjects to the procedure because of the abdominal incision; however, this is unlikely to have introduced bias (Fig. 3).
Detection bias
Blinding of outcome appraisal was performed in four studies [18, 23, 24, 27] (Fig. 3). Nonetheless, the NOS scores (Table 4) showed that almost all included cohort studies depended on patient records for assessment of outcomes.
Attrition bias
In six studies [18, 19, 22–24, 26], the follow-up rates were 80 % or more (Table 4, follow-up adequacy). Also, these studies had complete data, missing data were balanced across groups or were imputed using appropriate methods.
Reporting bias
Primary and secondary prespecified outcomes were reported in six papers [18, 19, 21, 22, 24, 27] (Fig. 3).
Other bias
One study [12] showed a low risk of bias due to funding and a conflict of interest, whereas five studies [19, 23–25, 27] showed a high risk of bias because of the source of funding or the presence of declared conflicts of interest (Fig. 3).
Confounders
Baseline characteristics able to act as confounders (age, body mass index, concurrent hysterectomy, other concurrent procedure or previous abdominal surgery) did not differ between groups in four studies [18, 20–22], and four studies [12, 23, 25, 26] used regression models to control for confounders.
Follow-up time
In six studies [10, 21, 23, 25, 27, 28], subjects were followed up for 12 months or more (Table 4, follow-up length).
Effectiveness of sacropexy for apical prolapse
Only four studies [19, 21, 23, 27] compared outcomes related to postoperative POP-Q assessments and cure rate (Table 5). Three studies [21, 23, 27] showed no significant differences in point-C POP-Q measurements 1 year after MISC or OSC. Freeman et al. [27] also found no significant difference in the satisfaction rate between MISC and OSC. Nosti et al. [12] found no significant difference in the apical prolapse recurrence rate at 1 year after controlling for confounding factors. These results are in accordance with those of other studies [21, 23, 25, 27, 28] (Table 5).
Complications of sacropexy
Nine studies compared complication rates between interventions [12, 19–21, 23, 25–28]. Complications were divided into intraoperative and postoperative. Events were extracted and included in the meta-analysis. Conversion to the open approach was not considered a complication. Two studies [12, 25] were not included in the meta-analysis because adverse events were reported as the sum of all complications. Overall complications (i.e. intraoperative plus postoperative) were similar between groups when combined in the meta-analysis (OR 0.91, 95 % CI 0.51 – 1.62, p = 0.74; Fig. 4). However, Nosti et al. [12] found a higher rate of overall complications with OSC (p < 0.01).
Intraoperative complications
Seven studies compared intraoperative complication rates between MISC and OSC (Fig. 5). The rate was 1 % for MISC and 2.4 % for OSC (Table 6). Although this favoured MISC, the difference was not statistically significant (OR 0.83, 95 % CI 0.51 – 1.34, p = 0.44). This is comparable to previously reported results [25] showing no significant differences between groups (p = 0.1014). Similarly, no significant differences were found when reported intraoperative complications were separately studied, including haemorrhage, bladder injury, intestinal injury and colpotomy (Appendices S1–S5). However, the transfusion rate was significantly lower with MISC (OR 0.41, 95 % CI 0.20 – 0.83, p = 0.01; Fig. 6).
Postoperative complications
Of nine studies for which postoperative complications were reported, seven were pooled in the meta-analysis (Fig. 7). The rate was 5.41 % for MISC and 10.02 % for OSC (Table 6). However, no significant differences were identified (OR 0.82, 95 % CI 0.48 – 1.42, p = 0.64).
No significant differences were found when postoperative complications were studied separately, including wound complications (disruption, infection, haematoma and hernia), fever, sepsis, thromboembolic events (deep venous thrombosis and pulmonary embolus), ileus/small-bowel obstruction/constipation, urinary retention, urinary infection, urinary incontinence, abdominal pain requiring analgesics, pulmonary complications (pneumonia, unplanned reintubation and failure to wean ventilation within 48 h), neurological complications (cerebrovascular accident, peripheral nerve injury and delirium) (Appendices S6–S16), and mesh erosion (Fig. 8). Similarly, Nosti et al. [12] found no significant differences in thromboembolic events, wound complications and mesh erosion.
Length of hospital stay
Of nine studies [12, 19–22, 24, 26–28] for which LOS was reported, only four could be combined in the meta-analysis (Fig. 9). LOS (expressed in days) was significantly shorter following MISC (MD −1.57 days, 95 % CI −1.91 – −1.23 days, p < 0.00001; Fig. 9). These results are in accordance with those of other studies [20–22, 24, 26] (all p < 0.001).
Estimated blood loss
Of nine studies [12, 19–22, 24, 27, 28] for which EBL was reported, only five were combined in the meta-analysis (Fig. 10). EBL was significantly greater with OSC than with MISC (MD −113.27 mL, 95 % CI −163.67 – −62.87 mL, p < 0.0001; Fig. 10). These results are in accordance with those of other studies [20–22, 24].
Operating time
OT was evaluated in ten studies [12, 18–22, 24, 26–28]. OT was defined differently among the studies so inclusion in the meta-analysis resulted in substantial heterogeneity. Consequently, a subgroup analysis was performed. “Room operating time” (i.e. total time in the operating theatre) was shorter with OSC [24, 28]. Similarly, “from incision to closure” OT was shorter with OSC in five studies [12, 19, 21, 24, 26]. Meta-analysis of two studies [12, 19] showed a significantly shorter OT with OSC (MD 87.47 min, 95 % CI 58.60 – 116.34 min, p < 0.0001; Fig. 11). In four studies [18, 20, 22, 27], OT was not specifically defined. Interestingly, none of them showed significant differences between procedures. However, in one study [22] this was attributed to the fact that OSC was probably used to treat more complex cases. Hoyte et al. [24] also evaluated the OT required only for sacropexy and found shorter OT with OSC (p < 0.001).
Postoperative pain
Postoperative pain was evaluated in three studies. Freeman et al. [27] evaluated morphine use over 3 days, whereas Collins et al. [18] measured the number of postoperative oral narcotics required. Although usage of postoperative analgesics appeared to be less with MISC, the difference did not reach statistical significance (SMD −0.41, 95 % CI −0.83 – 0.01, p = 0.05; Fig. 12). Similarly, Khan et al. [25] found no significant differences in perioperative pain between groups (p = 0.14).
Postoperative quality of life
Postoperative QoL was evaluated in three studies [18, 23, 27]. Two studies [18, 27] showed no significant differences between groups applying the Short Form-36 Health Survey. Similarly, applying the Prolapse QoL Questionnaire, one study [27] showed no significant differences between the approaches (p = 0.95). Additionally, no significant differences were found for the subscales of the PFDI-20 and PFIQ-7 questionnaires (p = 0.68) or the PISQ-12 questionnaire (p = 0.32) [23].
Mortality
In two studies [20, 26] fatalities occurred after MISC and OSC. In one study [20] one patient died in the open arm due to sepsis and subsequent multiorgan failure after a bowel perforation, and in the other [26] one patient died in each group, but the causes of death were not specified. When combined in the meta-analysis no significant differences were found (OR 0.44, 95 % CI 0.06 – 3.48, p = 0.44; Fig. 13).
Secondary analyses
Publication bias
Funnel plots were used to find the risk of publication bias (Appendices S17–S23). However, in cases of funnel plot asymmetry, further tests could not be done because fewer than ten studies were included in the meta-analysis for each outcome [9].
Sensitivity analyses
After removing observational studies with a low NOS score, the pooled estimate of effect remained similar between groups for all outcomes studied. Only the results related to “complications of sacropexy” would have changed if fixed-effects analysis had been performed instead. However, this would have placed excessive weight on one observational study [26] reducing the weight of the more methodologically rigorous study [27]. Studies with participation of experienced surgeons and surgeons in their learning curve were also compared. Studies in which concomitant hysterectomy was performed were also compared with those in which hysterectomy was not done. These comparisons revealed no differences between the surgical approaches, indicating that the results obtained are highly robust.
Discussion
Of the previous systematic reviews on this topic, the meta-analysis by Reza et al. [7] and the Cochrane review [8] only included one study each on sacrocolpopexy [19, 28], which were included in the present systematic review. Consequently, they are not discussed further. Additional consideration was given to the systematic review by Serati et al. [10]
Main findings
Twelve studies involving 4,757 participants compared MISC and OSC. The quality of the included studies was variable. Eleven studies were nonrandomized, and confounders were present in the majority. However, only three nonrandomized studies [20, 24, 28] had a low NOS score (five stars). It is clear though that poor methodological rigour in the included studies may have reduced the strength of the results, and cautious interpretation is advised. At 1 year postoperatively, MISC and OSC were equally effective in terms of point-C POP-Q measurements and recurrence rate. While mortality rate and the overall, intraoperative and postoperative complication rate favoured MISC, these differences did not reach statistical significance. Specifically, no significant differences were found in the mesh erosion rate. QoL appeared to be similar between MISC and OSC in the different questionnaires evaluated. MISC was also associated with less postoperative pain in terms of the quantity of postoperative analgesics. However, the difference was not statistically significant.
Strengths and limitations
Some weaknesses of this review must be addressed. Selection bias was inevitable due to the inclusion of nonrandomized studies. However, the scarcity of RCTs comparing MISC and OSC necessitated the inclusion of nonrandomized studies. Additionally, although articles in English, Portuguese and Spanish were sought, language bias is also present. This may have reduced the precision of the summary effect in the data analysis.
Many reasons for heterogeneity were detected: i.e. experience of surgeons, inclusion of concomitant hysterectomy and the differences in the techniques used among the studies. Specifically, regarding the outcome complication rate, performing hysterectomy at the time of promontofixation and the experience of surgeons were found to have an important influence on the results. Consequently, it could be argued that studies in which hysterectomy was concomitantly performed should have been excluded from this review. Nonetheless, it is believed that the trend seen among the studies is common in daily practice and therefore this review reflects real-world results.
Random-effects meta-analyses were performed when heterogeneity was high. Arguably, using analyses can exacerbate the risk of bias of smaller studies [9]. However, studies with larger populations were also less methodologically rigorous. Using random-effects analyses allowed the average of the effects to be obtained balancing the results more appropriately and giving more weight to studies with less risk of bias. Finally, it would have been valuable to explore results regarding the anterior and posterior compartments, postoperative stress urinary incontinence, costs and conversion rate between approaches, but this would have made the review unmanageable.
Despite these limitations, we believe that our results are valid. The comprehensive search used, the inclusion of trial registries in the search strategy and the attempts made to include unpublished studies would have reduced the risk of publication bias. Additionally, sensitivity analyses showed consistent results in the majority of outcomes. Furthermore, each step of the review was undertaken following a systematic approach based on the recommendations of The Cochrane Handbook [9] using a predefined protocol with strict inclusion and exclusion criteria as well as a thorough critical appraisal using appropriate assessment tools.
Interpretation
Regarding the effectiveness of MISC versus OSC, our results are in accordance with those found in a literature review [30] comparing LSC and OSC. A previous systematic review comparing RSC and OSC did not provide figures regarding anatomic outcomes but concluded that the two procedures were comparable [10]. The inclusion of a RCT in our review reinforces the existing assumptions showing clinical equivalence between MISC and OSC.
There was high heterogeneity in the overall complication rate. The causes were thought to be the varied experience of surgeons and the inclusion of concomitant hysterectomy, but sensitivity analyses do not support this. Nonetheless, one outlier was detected. The study by Klauschie et al. [21] showed a higher rate of complications with MISC. If this study was excluded from the group of experienced surgeons, MISC showed a significantly lower complication rate than OSC (p < 0.00001). This may suggest that the complication rate could be lower with MISC if experienced surgeons perform the procedure. A meta-analysis [31] comparing laparoscopy with laparotomy in benign gynaecological pathology showed a significantly lower overall complication rate with laparoscopy; however, the learning curve was not considered in that analysis. It is not clear why the complication rate reported by Klauschie et al. [21] differed from that in other similar studies. Claerhout et al. [32] found that satisfactory learning in LSC is achieved after 60 cases. None of the papers except two [20, 27] reported the number of procedures performed by surgeons. Future clarification in this regard may help to explore the influence of the learning curve on this and other outcomes.
Similar to the findings of Barber and Maher [30], this systematic review found that MISC was superior to OSC in terms of transfusion rate, LOS and EBL. Again, high heterogeneity was found in LOS and EBL. Reasons that might have influenced these outcomes are the number of other additional procedures performed concomitantly at the time of sacropexy, differences in population characteristics, differences among institutions in their policy on postoperative stay, preoperative stage of prolapse, and complexity of cases. Shorter LOS with laparoscopy was also found in a Cochrane review [33] of benign ovarian tumour and the review by Serati et al. [10] who also found shorter LOS and less EBL with RSC than with the open approach.
Similar to the findings of Barber and Maher [30], conflicting results were obtained regarding OT. Thorough analysis was not possible because clear definitions of OT were not provided for four studies. Additionally, the type of procedure performed, the preoperative stage of prolapse, and the varied findings during procedures possibly contributed to the heterogeneity observed. However, baseline characteristics in three studies [12, 24, 26] showed significantly more prior abdominal surgery in the open arm. This would have increased OT (due to potentially more adhesions). Nevertheless, OT in such studies were still shorter with OSC. Consequently, shorter OT was associated with OSC, as also found by Serati et al. [10] Conversely, MISC showed less postoperative pain in terms of the quantity of postoperative analgesics. Although this difference did not reach statistical significance, only two studies were available. More research regarding postoperative pain may confirm a statistically significant difference between surgical techniques for sacropexy similar to the findings in other conditions such as benign ovarian tumours [33] and endometrial cancer [34] in which postoperative pain is less with the laparoscopic approach.
Conclusions
Practical recommendations
Current evidence regarding sacropexy for the treatment of prolapse of the apical segment of the vagina indicates comparable anatomic outcomes between MISC and OSC at 1 year postoperatively. MISC was associated with a lower transfusion rate, shorter LOS and less EBL but also with longer OT. The rate of other complications was comparable between the approaches. MISC shows benefits over OSC and can be considered in clinical settings where technology and surgical proficiency are accessible. Cautious interpretation of the results is advised due to the high risk of bias.
Research recommendations
It is paramount to emphasize the importance of further high-quality research (RCTs) in order to minimize bias and reduce the effects of confounders. Similarly, precise definitions are required in future trials. The lack of clear concepts of OT limited the depth of the analysis. Furthermore, “surgeon’s expertise” may be defined to explore more accurately the influence of the learning curve on different outcomes. Alternatively, future studies may provide the approximate number of procedures that have been performed per approach by surgeons. Moreover, a supplementary systematic review evaluating results regarding the anterior and posterior compartments, postoperative stress urinary incontinence, costs, conversion rate and ergonomics will support the decision as to the best approach to sacropexy.
References
Barber MD, Maher C (2013) Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J 24(11):1783–1790
Toozs-Hobson P, Kelvin B, Cardozo L (1998) Management of vaginal vault prolapse. Br J Obstet Gynaecol 105:13–17
Turner DA, Shaw C, McGrother CW, Dallosso HM, Cooper NJ (2004) The cost of clinically significant urinary storage symptoms for community dwelling adults in the UK. BJU Int 93(9):1246–1252
Subramanian D, Szwarcensztein K, Mauskopf JA, Slack MC (2009) Rate, type, and cost of pelvic organ prolapse surgery in Germany, France, and England. Eur J Obstet Gynecol Reprod Biol 144(2):177–181
Maher CF, Feiner B, Baessler K, Schmid C (2013) Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev 4:CD004014
Yohannes P, Rotariua P, Pintoa P, Smitha A, Leea B (2002) Comparison of robotic versus laparoscopic skills: is there a difference in the learning curve? Urology 60(1):39–45
Reza M, Maeso S, Blasco JA, Andradas E (2010) Meta-analysis of observational studies on the safety and effectiveness of robotic gynaecological surgery. Br J Surg 97(12):1772–1783
Liu H, Lu D, Wang L, Shi G, Song H, Clarke J (2012) Robotic surgery for benign gynaecological disease. Cochrane Database Syst Rev 2:CD008978
Higgins J, Green S (eds) (2008) Cochrane handbook for systematic reviews of interventions. Wiley-Blackwell, Chichester
Serati M, Bogani G, Sorice P, Braga A, Torella M, Salvatore S et al (2014) Robot-assisted sacrocolpopexy for pelvic organ prolapse: a systematic review and meta-analysis of comparative studies. Eur Urol 66(2):303–318
Petticrew M, Roberts H (2006) Systematic reviews in the social science: a practical guide. Blackwell, Oxford
Nosti PA, Umoh AU, Kane S, White DE, Harvie HS, Lowenstein L et al (2014) Outcomes of abdominal and minimally invasive sacrocolpopexy: a retrospective cohort study. Female Pelvic Med Reconstr Surg 20(1):33–37
Pulliam SJ, Weinstein MM, Wakamatsu MM (2012) Minimally invasive apical sacropexy: a retrospective review of laparoscopic and robotic operating room experiences. Female Pelvic Med Reconstr Surg 18(2):122–126
Ross J, Preston M (2005) Laparoscopic sacrocolpopexy for severe vaginal vault prolapse: five-year outcome. J Minim Invasive Gynecol 12(3):221–226
McGovern DPB, Summerskill WSM, Valori RM, Levi M (2001) Key topics in evidence-based medicine. Bios, Oxford
Weber AM, Abrams P, Brubaker L, Cundiff G, Davis G, Dmochowski RR et al (2001) The standardization of terminology for researchers in female pelvic floor disorders. Int Urogynecol J Pelvic Floor Dysfunct 12:178–186
Cochrane Informatics & Knowledge Management Department (2014) RevMan.
Collins SA, Tulikangas PK, O’Sullivan DM (2012) Effect of surgical approach on physical activity and pain control after sacral colpopexy. Am J Obstet Gynecol 206:438.e1–e6
Geller EJ, Siddiqui NY, Wu JM, Visco AG (2008) Short-term outcomes of robotic sacrocolpopexy compared with abdominal sacrocolpopexy. Obstet Gynecol 112(6):1201–1206
Coolen AWM, van Oudheusden AMJ, van Eijndhoven HWF, van der Heijden TFM, Stokmans RA, Mol BJ, et al. (2013) A comparison of complications between open abdominal sacrocolpopexy and laparoscopic sacrocolpopexy for the treatment of vault prolapse. Obstet Gynecol Int. 2013:528636
Klauschie JL, Suozzi BA, O’Brien MM, McBride AW (2009) A comparison of laparoscopic and abdominal sacral colpopexy: objective outcome and perioperative differences. Int Urogynecol J Pelvic Floor Dysfunct 20(3):273–279
Elliott CS, Hsieh MH, Sokol ER, Comiter CV, Payne CK, Chen B (2012) Robot-assisted versus open sacrocolpopexy: a cost-minimization analysis. J Urol 187(2):638–643
Geller EJ, Parnell BA, Dunivan GC (2012) Robotic vs abdominal sacrocolpopexy: 44-month pelvic floor outcomes. Urology 79(3):532–536
Hoyte L, Rabbanifard R, Mezzich J, Bassaly R, Downes K (2012) Cost analysis of open versus robotic-assisted sacrocolpopexy. Female Pelvic Med Reconstr Surg 18(6):335–339
Khan A, Alperin M, Wu N, Clemens JQ, Dubina E, Pashos CL, Anger JT (2013) Comparative outcomes of open versus laparoscopic sacrocolpopexy among Medicare beneficiaries. Int Urogynecol J 24(11):1883–1891
Tyson MD, Wolter CE (2015) A comparison of 30-day surgical outcomes for minimally invasive and open sacrocolpopexy. Neurourol Urodyn 34(2):151–155
Freeman RM, Pantazis K, Thomson A, Frappell J, Bombieri L, Moran P et al (2013) A randomised controlled trial of abdominal versus laparoscopic sacrocolpopexy for the treatment of post-hysterectomy vaginal vault prolapse: LAS study. Int Urogynecol J 24(3):377–384
Paraiso MFR, Walters M, Rackley R, Melek S, Hugney C (2005) Laparoscopic and abdominal sacral colpopexies: a comparative cohort study. Am J Obstet Gynecol 192:1752–1758
Tyagi V, Hawthorn R, Guerrero K (2013) Sacrocolpopexy (SCP) - A Cohort Study Looking at Short, Medium and Long Term Outcome. Medical & Surgical Urology 2(118).
Barber MD, Maher C (2013) Apical prolapse. Int Urogynecol J 24:1815–1833
Chapron C, Fauconnier A, Goffinet F, Bréart G, Dubuisson JB (2002) Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynaecologic pathology. Results of a meta-analysis. Hum Reprod 17(5):1334–1342
Claerhout F, Roovers JP, Lewi P, Verguts J, De Ridder D, Deprest J (2009) Implementation of laparoscopic sacrocolpopexy – a single centre’s experience. Int Urogynecol J Pelvic Floor Dysfunct 20(9):1119–1125
Medeiros LR, Rosa DD, Bozzetti MC, Fachel JM, Furness S, Garry R, et al. (2009) Laparoscopy versus laparotomy for benign ovarian tumour. Cochrane Database Syst Rev 2:CD004751
de la Orden SG, Reza MM, Blasco JA, Andradas E, Callejo D, Pérez T (2008) Laparoscopic hysterectomy in the treatment of endometrial cancer: a systematic review. J Minim Invasive Gynecol 15(4):395–401
Acknowledgments
We acknowledge the valuable guidance of Andrea De Gouveia De Sa as a research assistant in the development of this paper and the team from Anglia Ruskin University library for their support in the database search and acquisition of the majority of the included papers.
Conflicts of interest
Maribel De Gouveia De Sa: Nothing to disclose
Leica Sarah Claydon: Nothing to disclose
Barry Whitlow: Nothing to disclose
Maria Angelica Dolcet Artahona: Nothing to disclose
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix S1
(DOC 32 kb)
Appendix S2
(DOC 31 kb)
Appendix S3
(DOC 31 kb)
Appendix S4
(DOC 31 kb)
Appendix S5
(DOC 31 kb)
Appendix S6
(DOC 32 kb)
Appendix S7
(DOC 31 kb)
Appendix S8
(DOC 31 kb)
Appendix S9
(DOC 31 kb)
Appendix S10
(DOC 31 kb)
Appendix S11
(DOC 31 kb)
Appendix S12
(DOC 31 kb)
Appendix S13
(DOC 31 kb)
Appendix S14
(DOC 31 kb)
Appendix S15
(DOC 31 kb)
Appendix S16
(DOC 31 kb)
Appendix S17
(DOC 26 kb)
Appendix S18
(DOC 31 kb)
Appendix S19
(DOC 26 kb)
Appendix S20
(DOC 26 kb)
Appendix S21
(DOC 26 kb)
Appendix S22
(DOC 26 kb)
Appendix S23
(DOC 30 kb)
Rights and permissions
About this article
Cite this article
De Gouveia De Sa, M., Claydon, L.S., Whitlow, B. et al. Laparoscopic versus open sacrocolpopexy for treatment of prolapse of the apical segment of the vagina: a systematic review and meta-analysis. Int Urogynecol J 27, 3–17 (2016). https://doi.org/10.1007/s00192-015-2765-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-015-2765-y