Abstract
Introduction and hypothesis
Botulinum toxin-A (BoNT-A) is a potent neurotoxin that is an effective treatment for patients with pharmacologically refractory detrusor overactivity (DO). Data assessing the effectiveness of trigonal BoNT-A are limited. This study evaluates adverse events (AEs) and short-term efficacy associated with trigonal and extratrigonal BoNT-A.
Methods
Electronic databases (PubMed, EMBASE, and the Cochrane database) were searched for studies comparing trigonal and extratrigonal BoNT-A for DO. Meta-analyses were performed using the random effects model. Outcome measures included incidence of AEs and short-term efficacy.
Results
Six studies describing 258 patients met the inclusion criteria. The meta-analysis did not show significant differences between trigonal and extratrigonal BoNT-A for acute urinary retention (AUR; 4.2 vs 3.7 %; odds ratio [OR]: 1.068, 95 % confidence interval [CI]: 0.239–4.773; P = 0.931) or high post-void residual (PVR; 25.8 vs 22.2 %; OR: 0.979; 95 % CI: 0.459–2.088; P = 0.956). The incidence of urinary tract infection (UTI; 7.5 vs 21.0 %; OR: 0.670; 95 % CI: 0.312–1.439; P = 0.305), haematuria (15.8 vs 25.9 %; OR: 0.547; 95 % CI: 0.264–1.134; P = 0.105) and post-operative muscle weakness (9.2 vs 11.3 %; OR: 0.587; 95 % CI: 0.205–1.680, P = 0.320) was similar in both groups. Finally, differences in short-term cure rates between two study arms were not statistically significant (52.9 vs 56.9 %; OR: 1.438; 95 % CI: 0.448–4.610; P = 0.542).
Conclusions
Although data are limited, no significant differences between trigonal and extratrigonal BoNT-A in terms of AEs and short-term efficacy were observed. Additional randomised controlled trials are required to define optimal injection techniques and sites for administering intra-vesical BoNT-A.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overactive bladder (OAB) is a syndrome that is characterised by urgency and frequency with or without urgency urinary incontinence (UUI). Its prevalence among the global population approaches 11 % and the female to male ratio is approximately 3:2 [1–4]. OAB is associated with significant decreases in health-related quality of life (QoL) and a diagnosis of detrusor overactivity (DO) may lead to upper genitourinary tract complications; particularly in neurogenic patients [5]. First-line treatment for OAB includes antimuscarinic agents for idiopathic detrusor overactivity (IDO) and a combination of antimuscarinic therapy and self-intermittent catheterisation (SIC) in neuropaths with neurogenic detrusor overactivity (NDO) associated with detrusor sphincter dysynergia (DSD) [3]. When these treatment modalities fail, intravesical injection with botulinum toxin-A (BoNT-A) offers an attractive alternative.
Botulinum toxin-A is a potent neurotoxin produced by Clostridium botulinum that is an effective, minimally invasive treatment option for patients with pharmacologically refractory IDO and NDO. The neurotoxin cleaves the synaptosomal-associated protein 25 (SNAP-25), which prevents the formation of the soluble N-ethylmaleimide attachment protein receptor (SNARE) complex that is required for neuromuscular transmission [1]. This inhibitory reaction is temporary as the toxin is ultimately inactivated and removed [1]. When BoNT-A is administered by urogynaecologists, the trigone of the bladder is typically spared owing to the potential risk of precipitating vesicoureteric reflux (VUR) from inhibition of the active trigonal antireflux mechanism [6, 7]. Despite this theory, the incidence of de novo VUR and other associated complications after injecting the trigone remains unknown and is poorly described in the literature. Therefore, the aims of the present study were two-fold. First, we aimed to compare the incidence of adverse events (AEs) in trigonal and extratrigonal BoNT-A in patients with DO. We also aimed to compare the short-term efficacy of both administration techniques by performing a meta-analysis of the relevant evidence.
Materials and methods
Literature search and study selection
A systematic search of PubMed and EMBASE was performed for all studies published relating to BoNT-A injections into and/or around the trigone of the urinary bladder in patients with DO by using the following in the search algorithm: (botulinum toxin-A or Botox® or Dysport®) and (trigone) and (bladder). The Cochrane Central Register of Controlled Trials was also searched for articles that investigated trigonal BoNT-A injections. The latest search was performed on 3 March 2014. Two authors (NFD and ER) independently examined the title and abstract of citations and the full texts of potentially eligible trials were obtained; disagreements were resolved by discussion. The reference lists of retrieved papers were further screened for additional eligible publications. When data were unclear or incomplete, the corresponding author was contacted to clarify data extraction. Case reports and case series with 5 or fewer patients were excluded and there were no language restrictions. Institutional review board was not sought as this study was a meta-analysis.
Eligibility criteria
Studies with data on trigonal BoNT-A injections were included. In addition, comparative studies between extratrigonal and trigonal BoNT-A were also included to compare the efficacy and frequency of AE for the purposes of meta-analysis. The primary end-points of the study were to determine the efficacy and safety of trigonal BoNT-A injections and to compare these parameters with those of extratrigonal BoNT-A injections. Short-term efficacy was defined by patients who were “subjectively/objectively dry” and by patients who demonstrated a “significant improvement in their symptoms” at the study’s first endpoint. Frequency of adverse events was determined by measuring the incidence of acute urinary retention (AUR), high post-void residual (PVR), straining to void, urinary tract infection (UTI), haematuria, de novo reflux and post-operative muscle weakness. All studies with no data on trigonal BoNT-A were excluded from the meta-analysis.
Data extraction and outcomes
The following information regarding each eligible trial was recorded: author’s names, journal, year of publication, study type, enrolment dates, follow-up protocol, total number of patients, patient demographics, indication for BoNT-A, type of BoNT-A injected, volume of BoNT-A injected, location and volume of intravesical injections, short-term efficacy and frequency of adverse events.
Statistical analysis
All pooled outcome measures were determined using a random effects model as described by DerSimonian and Laird [8] and the odds ratio (OR) was estimated with its variance and 95 % confidence interval (CI). The random effects analysis weighted the natural logarithm of each study’s OR by the inverse of its variance plus an estimate of the between-study variance in the presence of between-study heterogeneity. As previously described [9], heterogeneity between ORs for the same outcome between different studies was assessed. This was through the use of the I2 inconsistency test and Chi-square-based Cochran’s Q statistic test [10] in which P < 0.05 is taken to indicate the presence of significant heterogeneity. Analyses were conducted using StatsDirect version 2.5.6. (StatsDirect, Altrincham, Cheshire, UK).
Results
Eligible studies
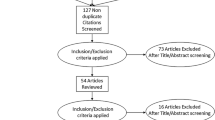
Six published studies containing data on intravesical trigonal injections with BoNT-A were identified; 4 of which directly compared trigonal and extratrigonal BoNT-A (Table 1) [2, 6, 11–14]. Four studies included data on patients with IDO refractory to anti-muscarinic therapy and 2 studies included data on NDO refractory to antimuscarinic therapy (Table 2). The initial search was performed based on the PRISMA statement and 49 articles were identified [15]. Eleven full text studies were assessed for eligibility; 5 of which were excluded (Fig. 1). Four studies were excluded as they did not contain data on trigonal BoNT-A injections and one editorial describing “injection techniques” was also excluded. All studies were published within the last 8 years and the spectrum of patients was reflective of modern clinical practice
A total of 258 patients were included in these studies, and data were available for analysis from 233 patients (152 in the trigonal arm and 81 in the extratrigonal arm). The mean age of the participants was 53 (25–76) years and 130 out of 233 (56 %) were female. There were no significant differences in terms of efficacy or frequency of adverse events between male and female patients (P = 0.7 and P = 0.64 respectively). None of the studies were multicentre trials and 2 trials were randomised, with details of the randomisation process described in both studies. Five studies included data on onabotulinum toxin-A and one study included data on abobotulinum toxin-A (Table 3). Indications for withdrawals were described in detail for the 3 studies in which patients withdrew. Two of the 6 studies were prospective without comparative data on extratrigonal BoNT-A injections [6, 12]. With the different study design taken into consideration, meta-analyses were performed with and without these two studies to enable comparison of outcomes with trigonal and extratrigonal injections.
Adverse events
Four studies describing 201 patients included comparative data on the frequency of acute urinary retention (AUR), high post-void residual (PVR), straining to void, urinary tract infection (UTI), haematuria, de novo vesicoureteric reflux and muscle weakness (Fig. 2) [6, 11, 13, 14]. Trigonal injections were associated with a non-significantly higher rate of AUR (trigonal versus extratrigonal: 4.2 % vs 3.7 %; OR: 1.068; 95 % CI: 0.239–4.773; P=0.931). In addition, trigonal injections were also associated with a non-significantly higher PVR (trigonal versus extratrigonal: 25.8 vs 22.2 %; OR: 0.979; 95 % CI: 0.459–2.088; P=0.956) and non-significantly higher incidence of straining to void (trigonal versus extratrigonal: 21 %.7 vs 14.8 %; OR: 1.394; 95 % CI: 0.617–3.150; P=0.424). Conversely, trigonal injections were associated with a non-significantly lower incidence of UTI (trigonal versus extratrigonal: 17.5 % vs 21.0 %; OR: 0.670; 95 % CI: 0.312–1.439; P=0.305) and haematuria (trigonal versus extratrigonal: 15.8 % vs 25.9 %; OR: 0.547; 95 % CI: 0.264–1.134, P=0.105). The incidence of post-operative muscle weakness was also non-significantly lower with trigonal injections (trigonal versus extratrigonal: 9.2 % vs 11.3 %; OR: 0.587; 95 % CI: 0.205–1.680; P=0.320). A meta-analysis on the incidence of de novo vesicoureteric reflux was not feasible as no patients developed de novo VUR in either study arm.
Meta-analysis of adverse events in trigonal vs extratrigonal BoNT-A. Each study is shown by the point estimate of the odds ratio (OR; square proportional to the weight of each study) and 95 % confidence interval (CI) for the OR (extending lines); the combined ORs and 95 % CIs by random effects calculations are shown as diamonds. a Trigonal vs extratrigonal BoNT-A and incidence of AUR (n = 201; P = 0.931; test for heterogeneity, Cochran Q = 0.0 [df = 1]; P = 0.959; inconsistency [I2] = 0 %). b Trigonal vs extratrigonal BoNT-A and incidence of high PVR (n = 201; P = 0.956; test for heterogeneity, Cochran Q = 0 [df = 1]; P = 0.984; I2 = 0 %). c Trigonal vs extratrigonal BoNT-A and incidence of straining (n = 201; P = 0.424; test for heterogeneity, Cochran Q = 0.1 [df = 1]; P = 0.746; I2 = 0 %). d Trigonal vs extratrigonal BoNT-A and incidence of UTI (n = 201; P = 0.305; test for heterogeneity, Cochran Q = 1.2 [df = 2]; P = 0.548; I2 = 0 %). Arrow indicates that 95 % CI extends beyond the depicted range. e Trigonal vs extratrigonal BoNT-A and incidence of haematuria (n = 201; P = 0.105; test for heterogeneity, Cochran Q = 0.8 [df = 3]; P = 0.859; I2 = 0 %). f Trigonal vs extratrigonal BoNT-A and incidence of muscle weakness (n = 201; P = 0.320; test for heterogeneity, Cochran Q = 0.8 [df = 1]; P = 0.381; I2 = 0 %)
Short-term efficacy
Three studies describing 179 patients included assessable comparative data on short-term cure rates associated with intravesical BoNT-A injections (Fig. 3) [6, 13, 14]. Short-term cure rates were defined as patients who were dry after their first follow-up investigations, which ranged from 3 to 8 weeks post-operatively (Table 3). Extratrigonal injections were associated with a non-significantly improved dry rate during the short-term follow-up period (trigonal versus extratrigonal: 52.9 % vs 56.9 %; OR: 1.438; 95 % CI: 0.448–4.610; P = 0.542). Four studies describing 201 patients included assessable comparative data on “significant improvement in symptoms” as the study’s endpoint [6, 11, 13, 14]. Extratrigonal injections were associated with a non-significant improvement in overall symptoms (trigonal versus extratrigonal: 59.8 % vs 67.9 %; OR: 0.703; 95 % CI: 0.344–1.437; P = 0.334).
Meta-analysis of short-term efficacy in trigonal vs extratrigonal BoNT-A. a Trigonal vs extratrigonal BoNT-A and overall short-term dry rates (n = 179; P = 0.542; test for heterogeneity, Cochran Q = 5.7 [df = 2]; P = 0.0058; I2 = 65 %). b Trigonal vs extratrigonal BoNT-A and overall symptomatic improvement (n = 201; P = 0.334; test for heterogeneity, Cochran Q = 0.4 [df = 1]; P = 0.527; I2 = 0 %)
Discussion
Initially, intravesical BoNT-A was described as a treatment option for DSD in 1988 and has since gained widespread acceptance as a reliable treatment option for IDO and NDO refractory to antimuscarinic therapy [16]. During the injection process the trigone is typically spared because of the theoretical risk of de novo VUR from inhibition of the peritrigonal anti-reflux mechanism. Notably, the main findings of the present review are that no significant differences in the frequency of AEs or short-term efficacy between trigonal and extratrigonal BoNT-A were demonstrated. Furthermore, no cases of de novo VUR were documented in studies that have investigated trigonal BoNT-A [2, 6, 11–14]. These findings may suggest that adrenergic control of trigonal smooth muscle may not be affected by the current doses of BoNT-A that are administered or that intravesical injections of BoNT-A in all sites within the urinary bladder may have similar denervation effects [2, 3, 6]. Admittedly, these conclusions are speculative owing to the paucity of comparative long-term data that are available on trigonal BoNT-A.
In general, the incidence of treatment-related AEs with BoNT-A is relatively low and the majority of complications are self-limiting. Our analysis is consistent with these features as a high PVR was the most frequent AE in both study arms (trigonal versus extratrigonal: 25.8 % versus 22.2 % respectively). It has previously been suggested that the incidence of AUR and high PVR is related to the dose of BoNT-A administered; however, our study may question this theory as the incidence of a high PVR was greatest in a study by Kuo et al. [2] in which 100 units of onabotulinum toxin-A was administered for IDO compared with doses of 200–300 units in the 4 remaining studies that evaluated onabotulinum toxin-A [6, 12–14]. It is apparent that additional randomised controlled trials will be required to clarify whether the dose of BoNT-A is a significant predictor of AUR. Furthermore, other investigators suggest that a multitude of additional factors such as injection technique, number of injections, equipment and type of anaesthesia may also play a role in the development of post-operative AUR and our results are consistent with these findings.
The short-term cure rate varied considerably in the studies assessed and ranged from 9 to 71 % in the trigonal arm compared with 31–70 % in the extratrigonal arm. In addition, overall symptom improvement also varied and ranged from 59 to 100 % in the trigonal arm compared with 63–100 % in the extratrigonal arm. The improvement noted in these parameters is consistent with previous randomised controlled trials that have compared BoNT-A with a placebo [17, 18]. Previously, it has been suggested that trigonal BoNT-A might provide greater short-term efficacy as sensory nerve endings are particularly dense in the trigone [12]. Inhibition of this region may improve the effect on bladder afferent pathways in patients with IDO and NDO. Notably, our analysis does not support or reject this hypothesis as no arm demonstrated significantly greater efficacy. However, it is arguable that the trigonal approach may ultimately be preferable to urogynaecologists because of the speed and ease of performance. Furthermore, patient tolerability does not appear to be an issue with trigonal BoNT-A as the agent was administered under local anaesthesia in two of the studies (Table 3) [5, 12].
Although the present study is the first meta-analysis to compare the AEs and short-term efficacy of trigonal and extratrigonal BoNT-A, there are several limitations and our findings should be viewed with caution. First, the number of studies included in the meta-analysis was relatively small owing to the paucity of comparative data available on trigonal and extratrigonal BoNT-A. Second, the volumes and formulations of intra-trigonal BoNT-A administered varied in each of the studies included and this variation may have influenced the frequency of AEs and short-term efficacy. However, it is noteworthy that there were no differences in the total dose or formulation of BoNT-A administered between the trigonal and extratrigonal arms of each individual study and an accurate comparison of AEs and efficacy was therefore achieved. Third, outcomes such as “high PVR” and “significant improvement in symptoms” were poorly defined in some studies, which may limit standardised comparisons between studies [2, 13]. Finally, the trigonal arm of the meta-analysis consisted of studies that injected the trigonal region exclusively as well as the bladder wall and peritrigonal region. This is a potential confounding variable and it is arguable that more definitive conclusions would have been achieved with direct comparisons between extratrigonal and exclusively trigonal/peritrigonal BoNT-A. At present, this type of meta-analysis is not feasible because of the paucity of comparative data available.
Conclusions
There remains significant controversy concerning the ideal site for administering intravesical BoNT-A. The present meta-analysis demonstrates no significant differences between trigonal and extratrigonal injections with regard to the frequency of AEs and short-term efficacy rates. However, we suggest that additional randomised controlled trials with standardised techniques and follow-up periods might be required to establish the most effective site of injection for intra-vesical BoNT-A.
Abbreviations
- AEs:
-
Adverse events
- AUR:
-
Acute urinary retention
- BoNT-A:
-
Botulinum toxin-A
- DO:
-
Detrusor overactivity
- DSD:
-
Detrusor sphincter dysynergia
- IDO:
-
Idiopathic detrusor overactivity
- NDO:
-
Neurogenic detrusor overactivity
- OAB:
-
Overactive bladder
- QoL:
-
Quality of life
- SIC:
-
Self intermittent catheterisation
- UTI:
-
Urinary tract infection
- VUR:
-
Vesicoureteric reflux
References
Mangera A, Andersson KE, Apostolidis A, Chapple C, Dasgupta P et al (2011) Contemporary management of lower urinary tract disease with botulinum toxin A: a systematic review of botox (onabotulinumtoxinA) and dysport (abobotulinumtoxinA). Eur Urol 60:784–795
Kuo HC (2011) Bladder base/trigone injection is safe and as effective as bladder body injection of onabotulinumtoxinA for idiopathic detrusor overactivity refractory to antimuscarinics. Neurourol Urodyn 30:1242–1248
Karsenty G, Baverstock R, Carlson K, Diaz DC, Cruz F et al (2014) Technical aspects of botulinum toxin type A injection in the bladder to treat urinary incontinence: reviewing the procedure. Int J Clin Pract 68:731–742
Ju R, Garrett J, Wu JM (2014) Anticholinergic medication use for female overactive bladder in the ambulatory setting in the United States. Int Urogynecol J 25:479–484
Tubaro A (2004) Defining overactive bladder: epidemiology and burden of disease. Urology 64:2–6
Karsenty G, Elzayat E, Delapparent T, St-Denis B, Lemieux MC et al (2007) Botulinum toxin type a injections into the trigone to treat idiopathic overactive bladder do not induce vesicoureteral reflux. J Urol 177:1011–1014
Schurch B, Schmid DM, Stohrer M (2000) Treatment of neurogenic incontinence with botulinum toxin A. N Engl J Med 342:665
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Dunne C, Burke JP, Morrow M, Kell MR (2009) Effect of margin status on local recurrence after breast conservation and radiation therapy for ductal carcinoma in situ. J Clin Oncol 27:1615–1620
Lau J, Ioannidis JP, Schmid CH (1997) Quantitative synthesis in systematic reviews. Ann Intern Med 127:820–826
Manecksha RP, Cullen IM, Ahmad S, McNeill G, Flynn R et al (2012) Prospective randomised controlled trial comparing trigone-sparing versus trigone-including intradetrusor injection of abobotulinumtoxinA for refractory idiopathic detrusor overactivity. Eur Urol 61:928–935
Mascarenhas F, Cocuzza M, Gomes CM, Leao N (2008) Trigonal injection of botulinum toxin-A does not cause vesicoureteral reflux in neurogenic patients. Neurourol Urodyn 27:311–314
Lucioni A, Rapp DE, Gong EM, Fedunok P, Bales GT (2006) Intravesical botulinum type A toxin injection in patients with overactive bladder: trigone versus trigone-sparing injection. Can J Urol 13:3291–3295
Abdel-Meguid TA (2010) Botulinum toxin-A injections into neurogenic overactive bladder–to include or exclude the trigone? a prospective, randomized, controlled trial. J Urol 184:2423–2428
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Dykstra DD, Sidi AA, Scott AB, Pagel JM, Goldish GD (1988) Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol 139:919–922
Herschorn S, Gajewski J, Ethans K, Corcos J, Carlson K et al (2011) Efficacy of botulinum toxin A injection for neurogenic detrusor overactivity and urinary incontinence: a randomized, double-blind trial. J Urol 185:2229–2235
Flynn MK, Amundsen CL, Perevich M, Liu F, Webster GD (2009) Outcome of a randomized, double-blind, placebo controlled trial of botulinum A toxin for refractory overactive bladder. J Urol 181:2608–2615
Acknowledgements
None
Funding
None.
Financial disclaimer/conflict of interest
None.
Author participation
Niall Davis: collection of data, manuscript writing; John Burke: statistical analysis; Elaine Redmond: collection of data; Saif Elamin: collection of data; Ciaran Brady: manuscript editing; Hugh Flood: project design, manuscript editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davis, N.F., Burke, J.P., Redmond, E.J. et al. Trigonal versus extratrigonal botulinum toxin-A: a systematic review and meta-analysis of efficacy and adverse events. Int Urogynecol J 26, 313–319 (2015). https://doi.org/10.1007/s00192-014-2499-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-014-2499-2