Abstract
Introduction and hypothesis
The aetiology of bowel incontinence in middle-aged women is multifactorial and the contribution of birth-related factors later in life is still poorly defined. The aim was to assess prevalence, risk factors and severity of faecal (FI, defined as the involuntary loss of faeces—solid or liquid) and anal incontinence (AI, includes FI as well as the involuntary loss of flatus) 20 years after one vaginal (VD) or one caesarean section (CS).
Methods
This was a registry-based national cohort study of primiparae giving birth in 1985–1988 and having no further births (n = 5,236). Data from the Swedish Medical Birth Register were linked to information from a pelvic floor disorder questionnaire in 2008 (response rate 65.2 %). Analysis of variance and multivariate analysis were used to obtain adjusted prevalence and odds ratios (adj-OR).
Results
Overall prevalences of FI and AI were 13.6 and 47.0 %. FI prevalence was higher after VD compared with CS [14.5 versus 10.6 %, adj-OR 1.43, 95 % confidence interval (CI) 1.16–1.77] but was not increased after acute versus elective CS. Perineal tear (≥second degree) increased the prevalence and risk of FI compared with no tear (22.8 versus 13.9 %, adj-OR 1.95, 95 % CI 1.33–2.85). The prevalence of FI was lower after VD with an episiotomy (11.1 %) and similar to that after CS (10.6 %). With each unit increase of current body mass index the odds of FI increased by 6 % (OR 1.06, 95 % CI 1.04–1.08).
Conclusions
Late FI and AI prevalences were higher after VD compared with CS. Perineal tear (≥second degree) versus no tear doubled the prevalence of FI. FI prevalence was similar after a CS and a VD combined with episiotomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The very thought of becoming permanently incontinent of faeces or flatus due to vaginal delivery (VD) has been shown to be one of the main reasons why some women contemplate caesarean section (CS) [1, 2]. In one study this attitude was most prevalent among female obstetricians [1] despite the fact that it has not been conclusively demonstrated that CS is protective against persistent faecal incontinence due to vaginal birth [3].
To date, much research has focused on sphincter injuries to explain faecal incontinence (FI) in parous women, based on the assumption that these injuries and their putative risk factors alone explain bowel incontinence after childbirth. This hypothesis has been challenged in recent studies that have shown that the vast majority of community-dwelling women with FI report that symptoms develop after 40 years of age and the main risk factors were not related to childbirth but instead to diarrhoea, irritable bowel syndrome, smoking, cholecystectomy and obesity [4]. Hence, these observations also hint at the essential fact that bowel continence is a complex bodily function with a multifactorial aetiology. The main components of bowel continence are: the quality of the colonic content; the integrity of the nervous and humoral control of intestinal motility and the endo- and exogenous secretory mechanisms of the gastrointestinal tract; the sensory function of the rectum and the anal canal; and finally the functional status of the pelvic floor muscles. The effect of pregnancy and vaginal birth on continence function affects mainly the last component mentioned [5].
Two reviews assessing whether CS is protective or not against bowel incontinence both concluded that it is not protective [3, 6]. However, according to the Cochrane Review the primary studies were methodologically poor with insufficient statistical power, employing different assessment tools and definitions, and had too short a follow-up time after delivery [3].
FI is a major public health matter of concern to women globally, and its occurrence in our rigorously toilet-trained society may seriously limit self-esteem and is disastrous socially and sexually for the afflicted individual. Therefore, the aim of this study was to assess the prevalence and identify risk factors for anal incontinence (AI) and FI in women 20 years after one VD or one CS.
Materials and methods
Women who participated in this study were identified from the Swedish Medical Birth Register at the Epidemiology Centre of the National Board of Health and Welfare in Sweden. Inclusion criteria for participation in this study were primiparae with one single birth 1985–1988 and no further births. Exclusion criteria were multiparity and multifetal or ongoing pregnancy. Obstetric data were combined with information from a postal questionnaire in 2008, 20 years after birth.
Study population, cohort characteristics and characteristics of the non-responders
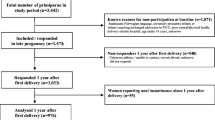
A description of the study population based on a flow chart and cohort characteristics has been presented and described in detail previously [7]. For ethical reasons, the Swedish Medical Birth Register does not disclose information about mode of delivery for non-responders. However, we requested information from the Medical Birth Register regarding the study population stratified according to body mass index (BMI) during pregnancy and fetal weight. Thus, we were able to compare responders and non-responders according to age, BMI and fetal weight. The non-responders were 1.6 years younger (49.6 ± 5.9 vs 51.2 ± 5.9 years; p < 0.001), and they were more often overweight or obese (37 vs 27 %; p < 0.001) and had an infant birthweight <4,000 g (43 vs 48 %; p < 0.003) compared to responders.
Women with a BMI <25 kg/m2 at delivery and women who had given birth to a child of <4,000 g were chosen at random from the total sample frame of women who had one single birth between 1985 and 1988 and no further births. All women with a BMI ≥25 kg/m2 and women who had given birth to a child weighing ≥4,000 g or had a recorded elective CS during the same calendar period were included in order to obtain sufficient numbers in these groups. Women were included regardless of maternal health status, gestational week and maternal/fetal complications for a greater generalization of results and therefore a more realistic basis for consultation.
A total of 10,117 women who fulfilled these criteria were identified from the Medical Birth Register. Addresses for 9,423 of these women could be traced in the Swedish Population Address Register, which includes all residents in Sweden. The 694 missing women included those newly deceased, women with an unknown address or hidden personal identity.
A letter was sent to 9,423 women who were asked to provide written, informed consent to participate and to complete an enclosed questionnaire regarding current pelvic floor function. After three mailing cycles during a 4-month period the questionnaire was returned by 6,148 women. Of these, 6,060 women were able to participate or gave their informed consent for participation. A further 824 women were excluded because they affirmed multiparity, or were multifetal or had an ongoing pregnancy at the time of the questionnaire. In this study the following numbers of women were excluded: 716 due to multiparity, 43 due to multifetal pregnancy, 6 due to ongoing pregnancy and 59 due to missing data about parity. In all, 5,236 women constituted the final study population, but a further 118 had missing data for important factors such as mode of delivery, so the final number was 5,118.
The questionnaire included 31 questions about current height and weight, urinary incontinence or AI and genital prolapse, menstrual status, hysterectomy, menopause and hormone treatment. FI was defined as the involuntary loss of faeces—solid or liquid. AI included these events as well as the involuntary loss of flatus (Fig. 1). Questions about soiling and urgency were not included in the questionnaire. We used the 5-item questionnaire of Jorge and Wexner [8] to classify incontinence as either absent (never) or the presence of each symptom of incontinence. This scale does not rank the three modes of incontinence in terms of severity. Instead the severity of incontinence was evaluated in terms of frequency of incontinence episodes for each type of leakage on a scale from 0 to 4 (0 = absent, 1 = <1/month, 2 = > 1/month but < 1/week, 3 = >1/week but <1/day and 4 = ≥1/day). When coping items such as need to wear a pad and the impact of incontinence on daily activities were included, the overall severity score ranged from 0 (continent) to 20 (complete incontinence). By mistake alternative 3 (>1/week but <1/day) was not included in our questionnaire. This does not affect the total score obtained. Total scores of 1–3 were defined as minor, scores 4–8 as moderate and scores 9–20 as severe. Scores ≥9 have been shown to indicate a significant impairment of quality of life [9].
Current BMI (kg/m2) was calculated from weight and height measurements reported in the questionnaire according to the WHO classification (normal weight 18.5–24.9 kg/m2, overweight 25.0–29.9 kg/m2 and obese ≥30 kg/m2). In this study a second-degree tear involves the fascia, muscles, perineal body but not the anal sphincter. A third-degree tear involves the anal sphincter and a fourth-degree tear extends through the rectal mucosa according to the WHO classification [10]. Tears (≥second degree) were handled as one group in the analysis of this study. In Sweden, during the calendar period 1985–1986, perineal tears were diagnosed by the midwife or the obstetrician according to the ICD-8 classification, and during 1987–1988 according to the ICD-9. During the 1980s, episiotomies were registered in the Medical Birth Register, but were not classified into medial or mediolateral. However, the prevailing trend in Sweden in 1985–1988 was to perform mediolateral episiotomy.
Statistical analysis
Statistical analysis was performed with SAS 9.1 (SAS Institute Inc., Cary, NC, USA). The chi-square test was used to compare categorical variables and Student’s t test to compare continuous variables. When original data were stratified, they were grouped according to the following: maternal age <23, 23–29, 30–34 and ≥35 years; BMI normal 18.5–24.9 kg/m2, overweight 25.0–29.9 kg/m2, obese 30 kg/m2 or higher; infant head circumference dichotomized to ≤35 or >35 cm; infant birthweight according to the most common stratification in the literature (starting at <3,000 g and with intervals of 500 g); and incongruity (which in this context indicates a disproportion between the size of the mother and child where probands with a VD were dichotomized into short mothers ≤160 cm who delivered a child ≥4,000 g and mothers who were also short at ≤160 cm, but delivered a child <4,000 g). Logistic regression analysis was used to demonstrate independent risk factors for AI/FI while controlling for potential risk factors and confounders. Potential risk factors used in the analysis were mode of delivery, maternal age at delivery, current BMI, infant birthweight and infant head circumference. The odds ratio (OR) and its 95 % confidence interval (CI) were calculated from the model. The Wald OR test was used to test for multiple parameters simultaneously. Adjusted prevalence was calculated using an analysis of variance after taking into account other risk factors [maternal age, infant birthweight, current BMI plus vacuum extraction (VE) for obstetric events during VD]. The OR and its 95 % CI were calculated from the logistic regression model. These variables were considered potential confounders or risk factors based on a combination of clinical considerations and the results from the logistic regression analysis. A p value of <0.05 was considered statistically significant. Non-linearity and threshold effects of the stratified variables (current BMI, infant birthweight and maternal age) were analysed for VD and CS separately. Subgroup analyses of the VD cohort were performed to address some specific obstetric events (tears, episiotomy, VE, disproportion). However, the number of forceps deliveries was too low (n = 24 of 758) to allow a meaningful analysis and was therefore excluded. The number needed to avoid AI/FI was calculated as the inverse of the absolute risk reduction, where risk reduction was the difference between the adjusted prevalence of VD compared with CS.
Results
The questionnaire was returned by 65.2 % (n = 6,148). The population of women included represents approximately 25 % of the total population of uniparae who delivered during 1985–1988 and had no further deliveries. A comparison of the basic characteristics of the women grouped according to mode of delivery has been published previously [7]. Women delivered by CS were older (p < 0.001) and gave birth to an infant with a lower birthweight (p < 0.001) and at an earlier gestational week (p < 0.001) compared to women after VD. The proportion of women with an age at delivery ≥ 35 years was higher (p < 0.001) in the CS group, whereas the proportion of infants with a birthweight ≥ 3,500 g was lower (p < 0.001) in the CS group compared to the VD group. The mean current age when answering the questionnaire was 53.7 years (SD 6.3) in the CS group and 50.4 years (SD 5.6) in the VD group. The mean follow-up was 21.5 years (SD 1.5) in the VD group and 21.8 years (SD 1.1) in the CS group.
The overall prevalence of FI and AI after only one birth was 13.6 and 47.0 %, respectively (Fig. 1). Figure 1 illustrates the distribution of the leakage modality, i.e. solid or liquid faeces, flatus or a combination of two or more. Leakage episodes less than once/month were most prevalent (liquid 75.5 %, solid 66.1 % and flatus 50.0 %). The proportion of women having daily leakage was 4.7 % for liquid (n = 31), 11.4 % for solid stool (n = 28) and 15.6 % (n = 364) for flatus, respectively.
The prevalence of AI was higher after VD compared with CS (48.3 vs 42.8 %, OR 1.25, 95 % CI 1.10–1.43) and also for FI (14.5 vs 10.6 %, OR 1.43, 95 % CI 1.16–1.77) (Table 1). These results indicate that 18 CS need to be performed to avoid 1 case of AI and 26 CS to avoid 1 case of FI. For each quality of leakage (solid, liquid and flatus, in any combination) the prevalence was higher after VD compared with CS, the difference being greatest for flatus incontinence (5.1 %, OR 1.23, 95 % CI 1.07–1.42) and smallest for solid stool (1.7 %, OR 1.54, 95 % CI 1.08–2.17). However, the proportion of women with isolated leakage of flatus differed little between women delivered by CS (74.1 %) or after a VD (70.6 %) (Table 1).
The proportion of women with severe incontinence, according to the Wexner Continence Grading Scale, was higher among vaginally delivered women compared with women delivered by CS (4.4 vs 2.8 %, OR 1.86, CI 1.03–3.58) (Table 2). There was, however, no difference in the adjusted prevalence and OR of AI after acute compared with elective CS [47.0 % (197/438) vs 43.0 % (336/760), OR 1.17, 95 % CI 0.92–1.49] or FI [12.7 % (54/438) vs 10.8 % (84/766), OR 1.20, 95 % CI 0.83–1.73].
The logistic regression analysis of AI and FI showed that VD, current BMI and maternal age were risk factors. The two strongest risk factors, VD and BMI, were associated with an almost doubled odds increase for FI compared with AI. For each unit increase of BMI there was an increased odds of AI by 3 % (OR 1.03, 95 % CI 1.02–1.04) and for FI by 6 % (OR 1.06, 95 % CI 1.04–1.08) (Table 3). In a subgroup analysis of both modes of delivery, it was found that BMI ≥30 in comparison with normal BMI was associated with an odds increase of approximately 50 % (for CS OR 1.57, 95 % CI 1.15–2.13; for VD OR 1.41, 95 % CI 1.18–1.67) (Table 4). Maternal age was a risk factor for both AI (an increase of 4 % yearly) and FI (3 % yearly). AI increased by 1 % for each 100 g increase in infant birthweight (OR 1.01, 95 % CI 1.00–1.02), but was not a risk factor for FI. Infant head circumference was not a risk factor (Table 3).
The prevalence of ≥second-degree tears was 4.6 % and a ≥second-degree tear was associated with an almost doubled prevalence of FI compared with women without ≥second-degree tears (22.8 vs 13.9 %, OR 1.95, 95 % CI 1.33–2.85) (Table 5). It was also possible to calculate the difference in OR for FI between surgically and vaginally delivered women including or excluding a perineal tear ≥second degree from the vaginal cohort. This resulted in a 4 % decrease in odds of FI, from 43 to 39 % (OR 1.39, 95 % CI 1.13–1.72). In this respect, it was possible to calculate the relative impact of ≥second-degree perineal tears (considering the rate of perineal tears of 4.6 % after vaginal birth) on the total risk increase of FI after VD. Excluding all ≥ second-degree perineal tears lowered the OR from 43 to 39 %, which meant that tears were responsible for only 9 % of the total risk increase after VD.
The rate of episiotomy in this study was 12.8 % (n = 510), which by international comparison is low, but similar to the national episiotomy rate (16 %) in Sweden in 1985 (Medical Birth Register). The prevalence of late FI after a birth with an episiotomy was 11.1 % compared to VD without episiotomy (14.7 %, OR 0.78, 95 % CI 0.58–1.05) (Table 5). The difference in prevalence of late FI between VD with an episiotomy and VD with tears (≥second degree) was 11.7 % units (OR 2.35, 95 % CI 1.43–3.83). VE (n = 734, 18.4 %) was a risk factor for both AI (OR 1.21, 95 % CI 1.03–1.42) and FI (OR 1.27, 95 % CI 1.02–1.58) compared with spontaneous VD (Table 5).
A comparison was also performed between women who had a VD with a second-degree tear and women who had an episiotomy in association with a VD. The prevalence of FI and AI, respectively, for women with a VD and second-degree tear was 10.0 and 42.0 % compared to 11.2 and 45.8 % for women with a VD and episiotomy (adjusted OR for FI 0.85, 95 % CI 0.48–1.54; adjusted OR for AI 0.88, 95 % CI 0.33–2.30).
In a subgroup analysis the combination of short mother ≤160 cm and infant birthweight ≥4,000 g compared with ≤160 cm and infant birthweight <4,000 g was not a risk factor for either AI (OR 1.01, 95 % CI 0.64–1.58) or FI (OR 0.81, 95 % CI 0.40–1.64).
Discussion
Main findings
Bowel incontinence affected a high percentage of women 20 years after one birth. FI and AI prevalences were higher after VD compared with CS and consistent for all qualities of any incontinence (flatus, liquid and solid stool). The prevalence of bowel incontinence did not differ between acute and elective CS. A greater proportion of women reported severe incontinence after VD compared with CS. Tears (≥second degree) almost doubled the prevalence of FI. FI prevalence after VD with episiotomy was similar to that after a CS.
Interpretations
The reported prevalence of AI and FI after childbirth varies considerably due to different definitions, types of questionnaires, selected populations and length of follow-up [12]. The overall late prevalences of FI and isolated flatus incontinence reported here are similar to those reported by Fritel et al. [11] (9.5 % for FI and 28.6 % for isolated flatus incontinence) taking into account the inclusion of nulliparous subjects in that study. Age is considered to be a major risk factor for FI [12] and was confirmed in this study, demonstrating an annual increase of FI by 3 %. The association between FI and age may not be linear, since FI usually has a late onset, with an incidence peaking at about 55 years [4] close to the mean age in this study (51 years). There was also an association between AI and age for both modes of delivery, and the effect of the independent risk factor age was greater in the CS group. Several studies [13] have shown BMI to be associated with incontinence, consistent with our findings. In a cross-sectional study of middle-aged women, Erekson et al. showed that for each 5-unit increase of BMI the risk of AI increased by 21 % [13]. Corresponding results in our study were 15 % for AI and 30 % for FI. Improvement of continence after weight reduction further indicates the importance of BMI for FI/AI [14].
To date, no studies have demonstrated any benefit of CS over VD on the long-term prevalence of bowel incontinence [3]. MacArthur et al. [15] showed that the prevalence of FI in women followed for 12 years was 11.5 % after spontaneous VD and 14.1 % after CS only. We, however, found a higher prevalence and risk after VD compared with CS, the difference being 6 % for AI and 4 % for FI. Large, homogeneous cohorts and late assessment may be needed to detect these differences, which also have been requested in one Cochrane Review [3]. The difference between VD and CS was further confirmed by results using the Wexner Continence Grading Scale. This scale was also used by Pinta et al. [16] who compared the outcome after CS vs VD in the short term. They demonstrated an almost doubled OR increase for severe incontinence, which is in agreement with our long-term findings. Our results also confirm earlier reports of no difference in FI between acute and elective CS [3].
The association between tears and incontinence is complex and largely unknown [4]. In this study ≥second-degree tears were associated with an almost doubled OR increase of FI despite the fact that the prevalence of ≥second-degree tears was low. As far as we are aware, this is the first time that tears have been shown to be associated with late incontinence. This can be interpreted that tears may be markers for occult injuries that become manifest later. Even if a tear is a bad omen for late incontinence, this study shows that the dominant factor for birth-related late FI is the trauma of VD in itself. This conclusion is supported by an electrophysiological study showing that neuronal damage to the pelvic floor may occur from a normal VD [17]. The incidence of ≥second-degree tears in Sweden (Medical Birth Register) has increased from 3.1 % (1987) to 16.9 % (2003) in primiparae, the aetiology of which is controversial. This trend is unsatisfactory since the prevalence of late FI associated with tears (≥second degree) is almost doubled in this study. Changes in the management of labour during the same period may explain this trend [18, 19].
To date, attention has focused on sphincter injuries assuming it to be the main risk factor for birth related FI, but results of studies in this field are contradictory [20]. Incontinence is common during the first months post-partum [21], but a majority with early problems recover [3]. The correlation between the extent of sphincter injury and severity of incontinence is poor [22]. Moreover, many patients with occult injuries at ultrasound are continent [23]. Sphincter injuries may be under-reported in our study since research has shown that 87 % of midwives and 27 % of junior doctors failed to recognize third to fourth-degree tears [24]. The rate of third-degree tears doubled to 15 % when all second-degree tears were reassessed [25]. It has been suggested that sphincter defects designated as occult are unrecognized clinical injuries, being < 1 %, if they exist at all [26].
Four cohort studies have investigated the association between episiotomy and symptoms of incontinence in the short term [27]. None of these found episiotomy to affect the risk of incontinence. Also in this study episiotomy was neither protective nor a risk factor, but the prevalence of FI after VD with episiotomy was 11.1 %, close to the 10.6 % after CS. The FI rate after a ≥second-degree tear (22.8 %) was doubled compared with VD plus episiotomy, the indication of which was mainly to avoid lacerations. In so far as episiotomy prevents the occurrence of a laceration, it is therefore highly protective for late FI in this group of women. In the 1980s in Sweden, episiotomy was used selectively and yet rates have been steadily decreasing from 21 % (1975) to 7 % (2005) [18]. During the same period the tear rates have increased due to a change in policy favouring spontaneous ruptures [27]. However, this restrictive use of mediolateral episiotomy was associated with a higher incidence of sphincter injuries [18, 28].
Interestingly, the proportion of isolated flatus incontinence did not differ between CS and VD. Numerically it was even higher after CS (74.1 vs 70.6 %). Further, the OR increase of FI was almost twice that of AI when comparing VD and CS (43 vs 25 %), although the prevalence of AI was based on a much larger group than FI (n = 2,425 vs 701). This indicates that isolated flatus incontinence mostly reflects bowel function, which has been postulated earlier [29], and that FI may be more sensitive to demonstrate birth-related pelvic floor dysfunction. Therefore, we suggest that FI may be the more specific outcome parameter to demonstrate birth-related incontinence after VD compared to AI in epidemiological studies.
Although several studies have shown VE to be a risk factor for sphincter injury [16, 30], none have demonstrated it to be a risk factor for incontinence, either in the short or in the long term [16]. The weak association between VE and FI in this study must be interpreted with caution, since many risk factors contributing to FI also affect the indication for a VE-assisted birth.
Strengths and weaknesses
The strengths and weaknesses of the design of this study have been discussed in detail previously [7]. The instrument to assess the quality, severity and impact of AI/FI in this report has been widely used [8]. Limitations to be considered are: Firstly, women with incontinence may be more predisposed to participate and AI/FI might therefore be overestimated. On the other hand, studies have shown that FI is embarrassing and not reported by more than every second person [29]. Secondly, leakage was self-reported. Thirdly, information on whether AI/FI was present or not before or/and during pregnancy or started after delivery is lacking. There is, however, scant evidence to suggest any difference in AI/FI prevalence before the first pregnancy or during pregnancy in women grouped according to mode of delivery. Fourthly, the length of the second stage of delivery is not documented in the Medical Birth Register and cannot be analysed.
Conclusion
Late FI and AI affect a large proportion of women 20 years after one delivery and are more prevalent after VD compared to CS. Episiotomy, if performed to avoid spontaneous lacerations, appears to be highly protective against FI and AI. A randomized controlled trial would be highly desirable to conclusively demonstrate this important clinical point. However, it is likely that evidence regarding the long-term effect of episiotomy on bowel continence function will largely rely on cohort studies as randomized controlled trials in this context are either inconceivable from an ethical point of view or in fact impracticable. Furthermore, we suggest that FI compared to AI may be the more specific outcome parameter to demonstrate birth-related incontinence after VD in epidemiological studies. The prevalence rates presented in our studies, however, constitute the effect of the first vaginal birth only and globally women today deliver on the average one or two more children, thereby adding further to these numbers. Incontinence has an enormous impact on women’s health, meriting greater attention and further studies to investigate the relative importance of the different components of a VD for the occurrence of FI.
References
Al-Mufti R, McCarthy A, Fisk NM (1997) Survey of obstetricians’ personal preference and discretionary practice. Eur J Obstet Gynecol Reprod Biol 73:1–4
Nygaard I (2005) Should women be offered elective cesarean section in the hope of preserving pelvic floor function? Int Urogynecol J Pelvic Floor Dysfunct 16:253–254
Nelson RL, Furner SE, Westercamp M, Farquhar C (2010) Cesarean delivery for the prevention of anal incontinence. Cochrane Database Syst Rev 2:CD006756
Bharucha AE, Zinsmeister AR, Schleck CD, Melton LJ 3rd (2010) Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology 139:1559–1566
Bharucha AE (2003) Fecal incontinence. Gastroenterology 124:1672–1685
Pretlove J, Thompson PJ, Toozs-Hobson PM, Radley S, Khana KS (2008) Does the mode of delivery predispose women to anal incontinence in the first year postpartum? A comparative systematic review. BJOG 115:421–434
Gyhagen M, Bullarbo M, Nielsen TF, Milsom I (2013) The prevalence of urinary incontinence 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or caesarean delivery. BJOG 120(2):144–151
Jorge JM, Wexner SD (1993) Etiology and management of fecal incontinence. Dis Colon Rectum 36:77–97
Rothbarth J, Bemelman WA, Meijerink WJHJ, Stiggelbout AM, Zwinderman AH et al (2001) What is the impact of fecal incontinence on quality of life? Dis Colon Rectum 44:67–71
World Health Organization (1996) International classification of diseases, 9th revision, clinical modification (ICD–9–CM). Geneva, Switzerland
Fritel X, Ringa V, Varnoux N, Zins M, Bréart G (2007) Mode of delivery and faecal incontinence at midlife: a study of 2,640 women in the Gazel cohort. Obstet Gynecol 110:31–38
Pretlove SJ, Radley S, Toozs-Hobson PM, Thompson PJ, Coomarasamy A, Khan KS (2006) Prevalence of anal incontinence according to age and gender: a systematic review and meta-regression analysis. Int Urogynecol J Pelvic Floor Dysfunct 17:407–417
Erekson EA, Sung VW, Myers DL (2008) Effect of body mass index on the risk of anal incontinence and defecatory dysfunction in women. Am J Obstet Gynecol 198:596.e1–596.e4
Cuicchi D, Lombardi R, Cariani S, Leuratti L, Lecce F, Cola B (2013) Clinical and instrumental evaluation of pelvic floor disorders before and after bariatric surgery in obese women. Surg Obes Relat Dis 9(1):69–75
MacArthur C, Glazener C, Lancashire R, Herbison P, Wilson D et al (2011) Exclusive caesarean section delivery and subsequent urinary and faecal incontinence: a 12-year longitudinal study. BJOG 118:1001–1007
Pinta TM, Kylänpää ML, Teramo KA, Luukkonen PS (2004) Sphincter rupture and anal incontinence after first vaginal delivery. Acta Obstet Gynecol Scand 83:917–922
Snooks SJ, Barnes PR, Swash M (1984) Damage to the innervation of the voluntary anal and periurethral sphincter musculature in incontinence: an electrophysiological study. J Neurol Neurosurg Psychiatry 47:1269–1273
Laine K, Gissler M, Pirhonen J (2009) Changing incidence of anal sphincter tears in four Nordic countries through the last decades. Eur J Obstet Gynecol Reprod Biol 146:71–75
Samuelsson E, Ladfors L, Wennerholm UB, Gåreberg B, Nyberg K, Hagberg H (2000) Anal sphincter tears: prospective study of obstetric risk factors. BJOG 107:926–931
Faltin DL, Otero M, Petignat P, Sangalli MR, Floris LA, Boulvain M et al (2006) Women’s health 18 years after rupture of the anal sphincter during childbirth: I. Fecal incontinence. Am J Obstet Gynecol 194:1255–1259
Kamm MA (1994) Obstetric damage and faecal incontinence. Lancet 344:730–733
Voyvodic F, Rieger NA, Skinner S, Schloithe AC, Saccone GT, Sage MR et al (2003) Endosonographic imaging of anal sphincter injury: does the size of the tear correlate with the degree of dysfunction? Dis Colon Rectum 46:735–741
Damon H, Henry L, Bretones S, Mellier G, Minaire Y, Mion F (2000) Postdelivery anal function in primiparous females: ultrasound and manometric study. Dis Colon Rectum 43:472–477
Sultan AH, Kettle C (2007) Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner D (eds) Perineal and anal sphincter trauma: diagnosis and clinical management, 1st edn. Springer, London, pp 13–19
Groom KM, Paterson-Brown S (2002) Can we improve on the diagnosis of third degree tears? Eur J Obstet Gynecol Reprod Biol 101:19–21
Andrews W, Sultan AH, Thakar R, Jones PW (2006) Occult anal sphincter injuries—myth or reality? BJOG 113:195–200
Hartmann K, Viswanathan M, Palmieri R, Gartlehner G, Thorp J Jr, Lohr KN (2005) Outcomes of routine episiotomy: a systematic review. JAMA 293:2141–2148
Räisänen S, Vehviläinen-Julkunen K, Gissler M, Heinonen S (2012) Hospital-based lateral episiotomy and obstetric anal sphincter injury rates: a retrospective population-based register study. Am J Obstet Gynecol 206:347.e1–347.e6
Whitehead WE, Wald A, Norton NJ (2001) Treatment options for fecal incontinence. Dis Colon Rectum 44:131–142
Wheeler TL, Richter HE (2007) Delivery method, anal sphincter tears and fecal incontinence: new information on a persistent problem. Curr Opin Obstet Gynecol 19:474–479
Ethical approval
Ethical approval was obtained from the Regional and the National Ethic Review Boards (the Ethics Committee at Sahlgrenska Academy, Gothenburg University, ref no.381-07, 13 August 2007 and the National Board of Health and Welfare, ref no. 34-9148/2007, 26 October 2007).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gyhagen, M., Bullarbo, M., Nielsen, T.F. et al. Faecal incontinence 20 years after one birth: a comparison between vaginal delivery and caesarean section. Int Urogynecol J 25, 1411–1418 (2014). https://doi.org/10.1007/s00192-014-2390-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-014-2390-1