Abstract
Introduction and hypothesis
There are several lower urinary tract dysfunctions (LUTD) that are more common in women than in men including incontinence, interstitial cystitis, and recurrent urinary tract infection. There is increasing evidence that these dysfunctions are associated with reduced blood flow, ischemia, hypoxia, and reperfusion resulting in free radical generation and oxidative damage. The goal of the current study was to determine if the level of circulating estrogen affects the response of the bladder muscle and mucosa to two in vitro models of oxidative stress: Incubation in the presence of hydrogen peroxide (H2O2) is the first model; the second is ischemia followed by reperfusion which results in the direct production of damaging free radicals. The motivation for this study is the current literature linking female-related LUTD with oxidative stress.
Methods
Eighteen female New Zealand white rabbits were divided into three groups: control, ovariectomized, and ovariectomized receiving continuous estrogen. Eight bladder strips from each of three rabbits per group were taken for in vitro ischemia/reperfusion (I/R) physiological experiments, while eight strips from the three remaining rabbit bladders per group were taken for in vitro H2O2 experiments. All tissue was analyzed for total antioxidant activity (AA) and malondialdehyde (MDA) levels. In addition, the organ bath buffer was also analyzed for AA.
Results
In vitro H2O2 was found to target the nerve, muscarinic receptor, and membrane equally causing more damage to bladder tissue than in vitro I/R. Ovariectomy resulted in lower contractility and higher lipid peroxidation. However, estrogen supplementation following ovariectomy protected the bladder against both models of oxidative stress by maintaining contractile responses to stimulation and decreasing lipid peroxidation.
Conclusions
The primary conclusion from this study is high estrogen protects the bladder from oxidative stress, whereas low estrogen makes the bladder more susceptible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are several lower urinary tract dysfunctions (LUTD) that are more prevalent in women than men. These conditions include interstitial cystitis (IC), recurrent urinary tract infections, and incontinence [1, 2]. The lower urinary tract includes the bladder which is composed of a thick smooth muscle wall and an inner tissue layer of mucosa (urothelium) which is intimately associated with lower urinary tract function [3, 4]. The mucosa is the first line of defense against bladder infections and normally prevents the penetration of urinary solutes into the bladder tissue wall. There is increasing evidence that incontinence, recurrent urinary tract infections, and IC are related directly to circulating estrogen levels. Specifically, relatively low estrogen levels decrease blood flow to the bladder muscle and mucosa resulting in ischemia and hypoxia. The result is a breakdown in mucosal integrity and ischemic damage to the muscle and mucosa [5, 6].
Prior assessments of the effects of altering circulating estrogen have demonstrated that increasing estrogen results in an increase in blood flow to the bladder muscle and mucosa, whereas low circulating estrogen results in decreased blood flow and significantly increased ischemia and hypoxia [5, 6]. Thus, low estrogen results in both increased permeability, ischemia, and free radical damage to cellular and subcellular membranes including the mitochondria, sarcoplasmic reticulum, and synaptic membranes [4–6].
It has been found that local blood flow to the mucosa in patients with IC is significantly reduced compared with non-IC patients during bladder distention [7, 8]. Clinically, the symptoms of pain and urgency of patients with IC are induced by distention and relieved by voiding of the bladder. We believe this is because ischemia and hypoxia of the bladder can affect sensory nerve membranes which lead to pain activation [9]. Our published studies have confirmed that in our rabbit model cyclical estrogen (2-week periods of high estrogen followed by 2-week periods of low estrogen) has significant effects on blood flow, ischemia, hypoxia, and mucosal damage consistent with the above observations [5, 6].
In the current study two physiologically relevant in vitro models of oxidative damage are utilized to characterize the effects of oxidative stress on the rabbit urinary bladder. The first is to expose isolated strips of bladder to ischemia/reperfusion (I/R, media equilibrated with nitrogen instead of oxygen without glucose in the media) for a period of time and then allowed to recover in the presence of oxygen and glucose. The second method is to subject isolated strips of bladder directly to increasing concentrations of hydrogen peroxide (H2O2) which would occur physiologically if the activity of superoxide dismutase (SOD) increased above the ability of catalase to neutralize the H2O2 which is the product of SOD activity [10, 11].
The specific aim of the current study was to determine the effect of I/R and direct exposure to H2O2 on the contractile responses of isolated strips of bladder body and base-urethra to different forms of stimulation, on the generation of malondialdehyde (MDA) in the bladder body and base-urethra, and on the total antioxidant activity (AA) levels of the bladder body, base-urethra, and physiological bath buffer.
Materials and methods
All methods were approved by the Institutional Animal Care and Use Committee of the Stratton VA Medical Center, Albany, NY, USA.
Animal methods
Eighteen adult female New Zealand white rabbits (approximately 3.5 kg each, 3–4 months old) were divided into three groups of six rabbits each. Group 1 (control) underwent sham surgery or received estrogen. Group 2 received bilateral ovariectomies and did not receive estrogen (Ovx). Group 3 received bilateral ovariectomies and had slow release estrogen tablets implanted in their subscapular area with a release of 1 mg/kg per day (Ovx + E). Estrogen implantation was simultaneous with ovariectomy. At the end of 2 weeks, each rabbit was anesthetized with 1 ml/0.2 ml/kg ketamine/xylazine by intravenous injection and was euthanized with 2 ml Fatal Plus Euthanasia fluid given IV. The bladder was rapidly removed and weighed. Five strips of the bladder body and three strips of the base-urethra from each bladder were taken for physiological testing. The balance of the bladder was separated by blunt dissection and frozen under liquid nitrogen and stored at −80 °C for biochemical analyses.
We did not measure the plasma estradiol levels in this experiment, but we did measure them in several previous experiments utilizing the identical models [12].
Physiological experiments
In vitro ischemia/reperfusion experiment
The bladders of three rabbits from each group were used and eight strips per rabbit (N = 24) were taken. The bladder was weighed and cut into full thickness equal length strips (about 1.5 cm long and 2 mm wide). The strips were placed in individual 15 ml organ baths containing Tyrode’s solution with glucose (1 mg/ml) at 36 °C and equilibrated with 95 % oxygen and 5 % carbon dioxide. The following experiment was performed:
Each strip was set at 2 g passive tension and stimulated with field stimulation (FS, 2 Hz, 8 Hz, 32 Hz, 1 ms duration for 20 s with 3 min in between stimulations). Carbachol (20 μM) and potassium chloride (120 mM) were then individually administered to each strip for 3 min with three washes with fresh buffer in between each stimulation. After the control stimulations, each bath was then switched to Tyrode’s solution without glucose and equilibrated in the presence of 95 % nitrogen and 5 % carbon dioxide for 1 h with stimulation at 32 Hz every 5 min. The buffer was then switched back to standard oxygenated Tyrode’s with glucose, and the strips were allowed to recover for 2 h. The strips were then stimulated as originally described and then cut, weighed, and frozen in liquid nitrogen for biochemical analyses. A sample of physiological buffer (1 ml) from each bath was taken after each stimulation and before and after the ischemic period and then frozen at −80° C for further biochemical analyses.
In vitro hydrogen peroxide experiment
The bladders of three rabbits from each group were used with eight strips per rabbit (N = 24) being taken. The tissues were stimulated by FS (2 Hz, 8 Hz, 32 Hz as in the I/R experiment), and carbachol (20 μM) was individually administered to each strip for a 3-min exposure. After each exposure, the strips were washed two times at 10-min intervals with fresh, oxygenated Tyrode’s solution. After the second wash, 0.1 % H2O2 was added for a 10-min incubation and the tissues stimulated again by FS and carbachol. This process was repeated for 0.2, 0.4, and 0.8 % H2O2. A sample of physiological buffer (1 ml) was taken after each carbachol stimulation and frozen at −80 °C for further biochemical analyses.
Biochemical analyses
CUPRAC assay
Total AA of the physiological buffer was quantified by the cupric ion reducing antioxidant capacity (CUPRAC) assay [13, 14]. For the CUPRAC assay, all samples and standards were prepared in duplicates in which 150 μl of the sample or standard was mixed with 150 μl each of 1 M ammonium acetate, 7.5 mM neocuproine, and 10 mM copper(II) chloride dihydrate. The standard curve was established using 1 mM L-ascorbic acid. The concentrations were 1,000, 500, 250, 125, 62.5, and 31.25 μM, as well as a blank (Tris buffer). Once the ammonium acetate, neocuproine, and copper(II) chloride dihydrate were added to the samples and standards, the test tubes were incubated at room temperature for 30 min. At this point, they were analyzed in a Hitachi U-2001 Spectrophotometer set to read at 450 nM [15–17].
Malondialdehyde assay
Malondialdehyde levels of the tissue and experimental strips were quantified using a thiobarbituric acid (TBA)-based assay [18, 19]. Malondialdehyde is the organic compound with the formula CH2(CHO)2. This reactive species occurs naturally and is a marker for oxidative stress. The following is a brief description of the assay: Tissues are homogenized in 50 mM Tris, pH 7.6 at 100 mg/ml; 625 μl of sample is mixed with 12.5 μl of FeSO4 in a 25-ml Erlenmeyer flask and incubated at 37 °C for the following times: 0, 30, 60, and 120 min. At each time point, 50 μl of each sample is removed and mixed with 15 μl of 40 % trichloroacetic acid (TCA) in duplicate to cause protein precipitation and aid with lipid extraction [20, 21]. All sets of samples are spun in a microcentrifuge for 2 min and 50 μl aliquots taken into 12 × 75 test tubes.
The standard triethoxypropane (TEP) is made as a 1 % v/v solution in diluent (200:60 mixture of KCl-Tris/40 % TCA). All samples and standards receive 750 μl of 1 % TBA pH 7.4 and undergo a 90 °C incubation for 30 min. The reaction yields a TBA-MDA adduct which is quantified by fluorometry at 534 nm EX and 553 nm EM.
Statistical analyses
One- and two-way analysis of variance were used to determine if significant differences were present among the groups, and the Tukey test was used to statistically compare individual groups. A p < 0.05 was required for statistical significance.
Results
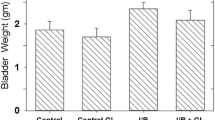
The average rabbit body weights (3.5 ± 0.6 kg) were the same for the three groups. The bladder weights for the control, Ovx, and Ovx + E groups were 2.5 ± 0.4, 1.8 ± 0.2, and 4.0 ± 0.5 g, respectively. The average bladder weight from rabbits in the Ovx group was significantly lower than control, and the average bladder weight from the Ovx + E group was significantly higher than both the control and Ovx rabbits.
Figure 1 shows the baseline contractile responses of the bladder body and base-urethra to FS and chemical/pharmacological stimulation for the three groups (control, Ovx, and Ovx + E). The Ovx group demonstrated significantly lower contractile responses to all forms of stimulation compared to the control and Ovx + E groups. For both types of tissue, the Ovx + E group showed significantly higher contractile responses to all forms of stimulation compared to the Ovx group.
Contractile force of isolated strips of bladder body (top) and bladder base-urethra (bottom) to FS, carbachol, and KCl. Each bar is the mean ± SEM of six individual rabbits. * = Significantly different from control; X = significantly different from Ovx; & = significantly different from bladder body; p < 0.05
The effects of H2O2 on the contractile responses of bladder body and base-urethra to FS at 32 Hz and to carbachol stimulation are presented in Figs. 2 and 3, respectively. The contractions are expressed as a percent of contraction without H2O2 at the varying concentrations of H2O2 (0.1, 0.2, 0.4, and 0.8 %). For both tissues, H2O2 produced a progressive and similar decrease in contractile responses to both forms of stimulation for the control and Ovx groups. The Ovx + E group for both types of tissues showed significantly higher contractile responses to both forms of stimulation at all concentrations of H2O2 when compared to the control and Ovx groups. Similar results were obtained for 2 and 8 Hz FS (data not shown).
It is important to note that chemical stimulation with KCl was not utilized in the H2O2 experiments to avoid having to wash the bladders between carbachol and KCl, thus increasing the exposure time to the H2O2.
Figure 4 shows the effect of I/R (as the percentage of the pre-I/R response). FS was significantly more sensitive to I/R than were carbachol and KCl. Control and Ovx tissues had very similar responses to I/R except for the base-urethral tissues where the responses of the Ovx group to 2 and 8Hz FS were significantly lower than the responses of the control tissue. All responses of the bladder body strips were significantly higher for the Ovx + E than for control and Ovx groups. For the base-urethral strips, the responses to 32 Hz FS, carbachol, and KCl were significantly higher for the Ovx + E group than for the other two groups. The responses of both tissues to carbachol for the Ovx + E group were equal to the response of the control tissue.
Effect of I/R on the contractile force of isolated strips of bladder body (top) and bladder base-urethra (bottom) to FS, carbachol, and KCl. Each bar is the mean ± SEM of six individual rabbits. * = Significantly different from pre-I/R; & = significantly different from control; X = significantly different from control and Ovx; # = significantly different from FS from the same group; p < 0.05
Figure 5 shows the MDA levels expressed as μM/mg protein for the bladder body and base-urethra for the three groups: exposed to H2O2 and I/R and for tissue not exposed to oxidative stress. MDA levels were significantly higher in all H2O2 and I/R strips compared to unexposed tissue. Unexposed tissue and strips had significantly higher MDA levels in the Ovx group compared to the control group for tissues. H2O2 produced higher levels of MDA compared to I/R for the bladder body but not for the base-urethra in both Ovx and Ovx + E groups. In general, all unexposed tissue and strips (body and base-urethra) had significantly higher MDA levels in the Ovx + E group compared to the control group. All MDA levels for the H2O2 and I/R strips in the Ovx + E group were significantly lower compared to those of the Ovx group but higher than the control group.
Effect of H2O2 and I/R on the MDA concentration of the bladder body (top) and bladder base-urethra (bottom). Each bar is the mean ± SEM of six individual rabbits. 1 = Tissue not exposed to either H2O2 or I/R; * = Significantly different from tissue, p < 0.05; x = significantly different from control, p < 0.05; # = significantly different from Ovx, p < 0.05; & = significantly different from H2O2, p < 0.05; @ = significantly different from bladder body, p < 0.05
The CUPRAC assay was performed on organ bath buffer samples at various points during the H2O2 and I/R experiments. Although the bath buffer was sampled at various times, only the AA of preexposure and at the highest concentrations of H2O2 (0.8 %) is shown. The AA concentrations of 0.1 and 0.2 % were equal to preexposure while the concentration at 0.4 % was approximately half of that shown for 0.8 %. These indicate that, for the bladder body and base-urethra, higher concentrations of H2O2 resulted in significantly higher levels of AA in the organ bath fluid compared to bath fluid without H2O2 (Fig. 6). This would indicate that at the higher concentrations of H2O2 there was significant tissue damage allowing the intracellular antioxidants to leak out into the organ bath. The ability of H2O2 to disrupt cellular membranes and tissues is well documented in the literature and is consistent with the data presented [22–24].
In the I/R experiment (Fig. 6), the bath fluid of the postischemia Ovx + E had significantly lower AA compared to preexposure for bladder body and base-urethra. Unlike the H2O2 experiments, I/R did not mediate an increase in the release of AA into the bath fluids. This is probably because the damage mediated by I/R is intracellular, thus maintaining the integrity of the plasma membrane.
Discussion
Estrogen has been indicated as a protective agent in a wide variety of pathological states including coronary heart disease, Alzheimer’s disease, and multiple sclerosis [25–27]. This protection includes, in part, its ability to reduce oxidative stress [28]. The present study investigated the protective role of estrogen in two in vitro models of oxidative stress.
A significant increase in average bladder weight for the estrogen-treated groups compared to the control and Ovx groups was expected. Previous studies have shown that estrogen increases rabbit bladder mass due to increased functional hypertrophy of smooth muscle which is accompanied by increased protein synthesis, angiogenesis, and muscle mass [29, 30]. Specifically, this increase in cell mass results in a type of “functional hypertrophy” in which contractile response to all forms of stimulation is improved and is accompanied by an increased ratio of smooth muscle to collagen [31]. Additionally, estrogen stimulates angiogenesis of the bladder, which increases vascular density and distribution which also increases bladder mass and mitigates ischemic effects [32].
The data from the contractility studies revealed that ovariectomy and estrogen supplement had different effects on baseline contractile responses to FS and carbachol. As was expected, ovariectomy decreased the baseline contractile response to each type of stimulation for the bladder body and base-urethra, while estrogen significantly increased the baseline contractile response. Therefore, without the additional stress of either I/R or H2O2, ovariectomy followed by estrogen supplementation protects against tissue damage mediated by the ischemia/hypoxia induced by Ovx and therefore improves detrusor contractility.
To summarize, we determined that estrogen supplementation increased the contractile responses of isolated strips of bladder body and base-urethra to FS, carbachol, and KCl. We found that I/R had a more negative impact on the contractile responses of isolated strips of bladder body and base-urethra to FS compared to carbachol or KCl stimulation. H2O2 had equally negative impacts on contractile responses to FS and carbachol stimulation.
Upon comparing the two different in vitro experiments, I/R inhibited contractile response to FS to a significantly greater degree than carbachol in all three groups. Conversely, different concentrations of H2O2 inhibited the response to both carbachol and FS equally. Carbachol is a muscarinic agonist which acts by stimulating its receptor directly without the participation of neurotransmission and requires only the receptor and the cell membrane. FS on the other hand mimics neurohumoral stimulation of muscle contraction through neurohumoral release, diffusion across the synapse, and stimulates postsynaptic receptors [6]. Based on these results, the I/R experiment targets the presynaptic innervation and therefore neuronal transmission to a greater extent than it does the postsynaptic targeted muscarinic receptor or the cell membrane, whereas H2O2 has both presynaptic (neurogenic) and postsynaptic (myogenic) effects.
Estrogen was found to be protective against both types of in vitro oxidative damage affecting contractility mechanisms. This finding is supported by the fact that in both experiments, the Ovx + E group exhibited significantly greater contractile responses to all forms of stimulation compared to the Ovx group for both the bladder body and base-urethra. Estrogen completely protected the bladder body and base-urethra in the I/R experiment when stimulated by carbachol indicating that any damage to the muscarinic receptor and the cell membrane was prevented. This may be due to improved blood flow because estrogen has been shown to have vasodilatory effects [33, 34]. Despite the specific mechanism, we know that estrogen supplementation provided protection against the oxidative damage to the receptor and cell membrane caused by I/R and the associated increase in free radical generation associated with short-term I/R. This indicates that mucosal and detrusor hypoxia was relieved and the mucosal permeability barrier was restored which was reflected by the full contractile response to carbachol [30, 35].
Although estrogen was found to significantly increase the contractile response at higher concentrations of H2O2,, the degree of protection was not as great as in the I/R experiment since the contractile response was only mildly restored. Therefore, estrogen was more successful in reversing the oxidative damage to the nerve compared to the more extensive damage to the cell membrane and muscarinic receptor caused by H2O2.. In future studies, it would be beneficial to establish whether increasing the dose of estrogen could reverse the apparent nerve damage caused by in vitro oxidative stress especially by H2O2 exposure.
The biochemical analyses revealed that MDA levels were significantly higher in the H2O2 and I/R experiments compared to nonexposed tissue across all groups for the bladder body and base-urethra. MDA is a biomarker for oxidative damage and a product of fatty acid lipid peroxidation induced by reactive oxygen species [18, 19, 36]. Therefore, in vitro exposure to H2O2 and I/R causes significant lipid peroxidation. This can be explained due to the ability of both models to generate free radicals although by different mechanisms. Ovariectomy for all unexposed tissue and strips caused significantly higher MDA levels compared to control which is consistent with the reduced contractile responses of this group.
Interestingly, H2O2 produced greater lipid peroxidation compared to I/R in the bladder body compared to the base-urethra in both Ovx and Ovx + E groups. Muscarinic receptor density is higher in the bladder body compared to the base-urethra [37]. These results may also indicate that H2O2 targets the muscarinic receptor to a greater degree than I/R does because there was greater lipid peroxidation in the bladder body compared to the base-urethra.
Lipid peroxidation is a commonly used measure of oxidative stress and is associated with a wide variety of degenerative diseases [38, 39]. MDA is one of the many end products formed during lipid peroxidation during which reactive oxygen species attack polyunsaturated fatty acids of cell membranes. Additionally, we saw differences in MDA levels from the bladder body and base-urethra in response to our two forms of in vitro oxidative stress. As far as we can ascertain, the differential responses of the bladder body and base-urethra have not been previously studied under these conditions.
There was significantly higher AA in the organ bath fluid at higher H2O2 concentrations (0.4 and 0.8 %) compared to preexposure levels. These results were expected because H2O2 is known to break down cell membranes [22–24]. Total AA of the bath fluid was not increased after I/R in any of the three groups.
One limitation of this study is the ability to correlate the data generated with in vivo oxidative stress. The data presented in this study would imply that H2O2 and I/R have different mechanisms by which they produce oxidative stress and bladder tissue dysfunction. However, both H2O2 and I/R do occur in vivo and studies have demonstrated similar findings using in vivo studies, although in these in vivo studies it would be very difficult to determine the relationship of the bladder dysfunctions observed with H2O2 or I/R mediated oxidative stress [5, 6, 29, 30, 40].
References
Sullivan MP, Yalla SV (2002) Physiology of female micturition. Urol Clin North Am 29(3):499–514, vii
Dörflinger A, Monga A (2001) Voiding dysfunction. Curr Opin Obstet Gynecol 13(5):507–512
Levin RM, Haugaard N, Hypolite JA, Wein AJ, Buttyan R (1999) Metabolic factors influencing lower urinary tract function. Exp Physiol 84(1):171–194
Hypolite JA, Longhurst PA, Gong C, Briscoe J, Wein AJ, Levin RM (1993) Metabolic studies on rabbit bladder smooth muscle and mucosa. Mol Cell Biochem 125(1):35–42
Palmieri K, Mannikarottu AS, Chichester P, Kogan B, Leggett RE, Whitbeck C, Levin RM (2007) The effects of cyclical oestrogen on bladder and urethral structure and function. BJU Int 99(1):171–176
Rehfuss A, Schuler C, Maxemous C, Leggett RE, Levin RM (2010) Cyclical estrogen and free radical damage to the rabbit urinary bladder. Int Urogynecol J 21(4):489–494
Irwin P, Galloway NT (1993) Impaired bladder perfusion in interstitial cystitis: a study of blood supply using laser Doppler flowmetry. J Urol 149:890–892
Pontari MA, Hanno PM, Ruggieri MR (1999) Comparison of bladder blood flow in patients with and without interstitial cystitis. J Urol 162(2):330–334
Chughtai B, Levin RM, Chichester P, Schuler C, Leggett RE, Mannikarottu A, De E (2008) Ischemic etiology of incontinence in women: a theory and a review. Open Urol Nephrol J 1:1–8
Agartan CA, Leggett RE, Kogan BA, Levin RM (2007) Effect of age on the response to in vitro ischemia and reperfusion of the rabbit bladder. Urol Int 78(2):155–159
Venugopal V, Leggett RE, Schuler C, Levin RM (2010) Effect of hydrogen peroxide on rabbit urinary bladder citrate synthase activity in the presence and absence of a grape suspension. Int Braz J Urol 36(6):749–757, discussion 757–748
Hass MA, Nichol P, Lee L, Levin RM (2009) Estrogen modulates permeability and prostaglandin levels in the rabbit urinary bladder. Prostaglandins Leukot Essent Fatty Acids 80(2–3):125–129
Bean H, Radu F, De E, Schuler C, Leggett RE, Levin RM (2009) Comparative evaluation of antioxidant reactivity within obstructed and control rabbit urinary bladder tissue using FRAP and CUPRAC assays. Mol Cell Biochem 323(1–2):139–142
Bean H, Schuler C, Leggett RE, Levin RM (2010) Antioxidant levels of common fruits, vegetables, and juices versus protective activity against in vitro ischemia/reperfusion. Int Urol Nephrol 42(2):409–415
Ozyürek M, Bektaşoğlu B, Güçlü K, Apak R (2009) Measurement of xanthine oxidase inhibition activity of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (CUPRAC) method. Anal Chim Acta 636(1):42–50
Apak R, Güçlü K, Ozyürek M, Karademir SE, Altun M (2005) Total antioxidant capacity assay of human serum using copper(II)-neocuproine as chromogenic oxidant: the CUPRAC method. Free Radic Res 39(9):949–961
Apak R, Güçlü K, Ozyürek M, Karademir SE (2004) Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem 52(26):7970–7981
Conti M, Morand PC, Levillain P, Lemonnier A (1991) Improved fluorometric determination of malonaldehyde. Clin Chem 37(7):1273–1275
Wasowicz W, Nève J, Peretz A (1993) Optimized steps in fluorometric determination of thiobarbituric acid-reactive substances in serum: importance of extraction pH and influence of sample preservation and storage. Clin Chem 39(12):2522–2526
Wade CR, van Rij AM (1988) Plasma thiobarbituric acid reactivity: reaction conditions and the role of iron, antioxidants and lipid peroxy radicals on the quantitation of plasma lipid peroxides. Life Sci 43(13):1085–1093
Lin AT, Yang CH, Chen KK, Chang LS (2005) Detrusor mitochondrial lipid peroxidation and superoxide dismutase activity in partial bladder outlet obstruction of rabbits. Neurourol Urodyn 24(3):282–287
Schalkwijk J, van den Berg WB, van de Putte LB, Joosten LA (1987) An experimental model for hydrogen peroxide induced tissue damage: effect on cartilage and other articular tissues. Int J Tissue React 9(1):39–43
Schalkwijk J, van den Berg WB, van de Putte LB, Joosten LA (1986) An experimental model for hydrogen peroxide-induced tissue damage. Effects of a single inflammatory mediator on (peri)articular tissues. Arthritis Rheum 29(4):532–538
Gough DR, Cotter TG (2011) Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell Death Dis 2:e213
Barrett-Connor E, Bush TL (1991) Estrogen and coronary heart disease in women. JAMA 265(14):1861–1867
Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E (1997) A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology 48(6):1517–1521
Garay L, Gonzalez Deniselle MC, Gierman L, Meyer M, Lima A, Roig P, De Nicola AF (2008) Steroid protection in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Neuroimmunomodulation 15(1):76–83
Stirone C, Duckles SP, Krause DN, Procaccio V (2005) Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol 68(4):959–965
Badger WJ, Whitbeck C, Kogan B, Chichester P, Levin RM (2006) The immediate effect of castration on female rabbit bladder blood flow and tissue oxygenation. Urol Int 76(3):264–268
Parekh MH, Chichester P, Lobel RW, Aikawa K, Levin RM (2004) Effects of castration on female rabbit bladder physiology and morphology. Urology 64(5):1048–1051
Lin AD, Levin R, Kogan B, Whitbeck C, Chichester P, Sokol R, Mannikarottu A (2006) Estrogen induced functional hypertrophy and increased force generation of the female rabbit bladder. Neurourol Urodyn 25(5):473–479
Lin AD, Mannikarottu A, Kogan BA, Whitbeck C, Chichester P, Leggett RE, Levin RM (2006) Estrogen induces angiogenesis of the female rabbit bladder. J Endocrinol 190(2):241–246
Juan YS, Mannikarottu A, Kogan BA, Leggett RE, Whitbeck C, Chichester P, Lin WY, Johnson A, Levin RM (2008) The effect of low-dose estrogen therapy on ovariectomized female rabbit bladder. Urology 71(6):1209–1213
Walker A, Tanner MJ, Husson P, Schuler C, Kogan BA, Buttyan R, Levin RM (2009) Differential expression of vascular endothelial growth factor, and angiopoietin 1 and 2 in functionally divergent experimental rabbit models of bladder hypertrophy. J Urol 181(6):2790–2796
Aikawa K, Sugino T, Matsumoto S, Chichester P, Whitbeck C, Levin RM (2003) The effect of ovariectomy and estradiol on rabbit bladder smooth muscle contraction and morphology. J Urol 170(2 Pt 1):634–637
Jo C, Ahn DU (1998) Fluorometric analysis of 2-thiobarbituric acid reactive substances in turkey. Poult Sci 77(3):475–480
Clemens JQ (2010) Basic bladder neurophysiology. Urol Clin North Am 37(4):487–494
Niki E (2013) Biomarkers of lipid peroxidation in clinical material. Biochim Biophys Acta
Radu F, Leggett RE, Schuler C, Levin RM (2011) The effect of in vitro ischemia/reperfusion on contraction, free fatty acid content, phospholipid content, and malondialdehyde levels of the rabbit urinary bladder. Mol Cell Biochem 346(1–2):179–186
Damaser MS, Whitbeck C, Chichester P, Levin RM (2005) Effect of vaginal distension on blood flow and hypoxia of urogenital organs of the female rat. J Appl Physiol 98(5):1884–1890
Acknowledgments
This material is based upon work supported in part by the Office of Research and Development Department of Veterans Affairs and in part by the Capital Region Medical Research Foundation.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malone, L., Schuler, C., Leggett, R.E. et al. Effect of estrogen and ovariectomy on response of the female rabbit urinary bladder to two forms of in vitro oxidative stress. Int Urogynecol J 25, 791–798 (2014). https://doi.org/10.1007/s00192-013-2289-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-013-2289-2