Abstract
Introduction and hypothesis
We compared hands-on manual perineal protection (MPP) and hands-off delivery techniques using the basic principles of mechanics and assessed the tension of perineal structures using a novel biomechanical model of the perineum. We also measured the effect of the thumb and index finger of the accoucheur’s dominant-posterior hand on perineal tissue tension when a modified Viennese method of MPP is performed.
Methods
Hands-off and two variations of hands-on manual perineal protection during vaginal delivery were simulated using a biomechanical model, with the main outcome measure being strain/tension throughout the perineal body during vaginal delivery.
Results
Stress distribution with the hands-on model shows that when using MPP, the value of highest stress was decreased by 39 % (model B) and by 30 % (model C) compared with the hands-off model A. On the cross section there is a significant decrease in areas of equal tension throughout the perineal body in both hands-on models. Simulation of the modified Viennese MPP significantly reduces the maximum tension on the inner surface of the perineum measured at intervals of 2 mm from the posterior fourchette.
Conclusions
In a biomechanical assessment with a finite element model of vaginal delivery, appropriate application of the thumb and index finger of the accoucheur’s dominant-posterior hand to the surface of the perineum during the second stage of delivery significantly reduces tissue tension throughout the entire thickness of the perineum; thus, this intervention might help reduce obstetric perineal trauma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vaginal delivery can lead to perineal trauma, which can cause pain [1] and infection [2] in the short term and pelvic organ prolapse (POP) and stress urinary incontinence (SUI) [3] in the long term. A number of measures have been explored to avoid/reduce such trauma, including episiotomy [4] and perineal support. In the past, perineal support during the time of the delivery of the fetal head was standard practice. Several different guiding techniques for protecting the perineum during the second stage of delivery have been suggested, but no consensus for definition of manual perineal protection exists. Manual perineal protection can be understood as the use of either one or two hands when the fetal head is crowning. Flexion or extension techniques have been suggested for the accoucheur’s left-anterior hand. Three main procedures were described in 1889 [5] to support the perineum using the accoucheur’s right-posterior, dominant hand: the central perineal support [5], the Viennese method [5], and Ritgen maneuver [6]. In the central support, the palm is firmly applied to the perineum in the midline, with no coordinative work mediated by fingers [5]. In the Viennese technique, the fingers are placed alongside the fourchette and vaginal opening [5]. In the Ritgen maneuver, the tips of four fingers are placed on the posterior perineum, behind the anus, and execute a forward pressure on the fetal chin to extend the fetal head [6]. In some countries, the practice of routine manual perineal protection has fallen out of favor and seems to have disappeared in recent years.
In the USA, evidence-based guidelines for managing the second stage of labor do not recommend routine performance of MPP [7]. In the UK, both the hands-on MPP or the hands-off delivery techniques are considered appropriate for facilitating spontaneous vaginal delivery [8, 9], and a recent survey showed that a majority of junior midwives prefer the hands-off delivery technique [10] based on results of two randomized trials [11, 12]. These randomized studies compared different MPP techniques and showed no beneficial effect on the rate or degree of perineal injury. However, the hands-on technique was insufficiently defined [11–13]. The studies failed to describe whether the purpose of the light pressure (executed by the anterior hand) was merely to slow down the passage of the fetal head through the perineal structures or if it should, in addition, maintain flexion [11, 12]. The terms “guarding” and “supporting” the perineum (executed by the right-posterior hand) were not defined or explained in detail [11, 12].
Lacking a technical tool to measure changes in perineal tissue tension during delivery has made it difficult to evaluate the effects of using MPP. Recently, an analytical study showed that the maximum perineal transverse strain is more than four times higher than the highest maximum anteroposterior strain and that 1 cm of the perineal tissue at the fourchette is transversely stretched to 2.77 cm in the final phase of the second stage of delivery [14]. Derived from the principles of mechanics, reduced perineal tension can be achieved by redistributing and spreading maximum tension over a larger area. A biomechanical model allowing depiction of displacements and stresses in tissue is being developed to measure alterations on perineal tissue tension during the simulation of vaginal delivery.
The aim of this study was to evaluate whether the modified Viennese method with fingers applied to perineal skin can reduce perineal tension throughout the entire thickness of the perineum when compared with the hands-off delivery technique. The goal of this testing was to evaluate the role of the thumb and index finger of the accoucheur’s dominant-posterior hand during the modified Viennese method of MPP. This study is a part of a larger project: Perineal Trauma Prevention, Evaluation, Education, and Recognition Study Group: Perineal Protection Program Incorporating the Principles of Physics (PEERS 5P’s project).
Methods
Developing the biomechanical model
To design this model, the initial geometry of the female pelvic floor at the beginning of the second stage of labor was based on available data from previous experimental, clinical, and biomechanical studies. The following anatomical and mechanical parameters were chosen to define the biomechanical model in order to correspond more accurately with dynamic changes in perineal anatomy during the final stage of labor: location and dimensions (length, thickness, angle) of the perineal structures (e.g. pubis, subpubic angle, inferior pubic rami, genital hiatus, perineal body, anus), fetal-head dimensions, trajectory of fetal head passage, location of the thumb and the index finger on the perineal surface, area of contact between fingers and perineum, coordinated movement between fingers, together with its vector and experimental data obtained from previous clinical measurements [15–28], and stereophotogrammetry performed during the second stage of labor [14]. The initial perineal body length (distance between the posterior margin of the hymen and the anterior margin of the anus) was set at 3.7 cm [15, 16] with a potential to stretch to 5.0 cm [17]. The thickness of the perineal body (the craniocaudal diameter in the sagittal plane in the midline) was set at 3 mm at the posterior margin of the hymen and 14 mm at the site of the external anal sphincter [18, 19]. The subpubic angle was selected at 90° [20–22]. The anteroposterior diameter of the pelvic outlet was 11.5 cm [21]. The chosen intertuberous diameter was 11 cm [22]. All these dimensions defined the length of the inferior puboischial rami at 7 cm. The chosen diameter of the genital hiatus was 3 cm. This was derived from the diameter of the levator hiatus [23–26], the length of the genital hiatus [27], and the circumference of the two fingers of the accoucheur being used for the routine gynecological examination. The chosen diameter of the molded fetal head was 9.5 cm [25, 28], and the chosen trajectory of fetal head passage through the birth canal followed the curve of Carus. Based on the available scientific data, a numerical finite element model of the perineum was created. Model geometry and mesh were created with HyperMesh software [29]; simulations were performed using Pam-Crash software [30].
The 3D mesh was composed of 162,310 tetrahedral elements (elements composed of four triangular faces). The mean edge size of the elements was 2 mm. Elastic, viscoelastic, or hyperelastic material models are usually used for soft biological tissue [31]. The perineal tissue undergoes extremely large deformations during vaginal delivery [14]. Therefore an elastic material model was not suitable. The finite element model was designed for slow and long processes that can be performed by quasistatic simulations. Response of viscoelastic materials is time dependent, but the final phase of the vaginal delivery takes minutes, which is considered to be a long time. A hyperelastic material model allows for large deformations and is not time dependent, which allows for shortening of the simulation without compromising the correct material response. Therefore, we used the quasi-incompressible, transversely isotropic, hyperelastic Mooney–Rivlin material model for soft tissue. Strain energy density function for this material is:
where I1, I2, I3 are the invariants of the right Cauchy–Green deformation tensor.
The 2nd Piola–Kirchhoff stress tensor is obtained as follows:
where εij is the Green–Lagrange strain tensor.
Coefficients A and B are material parameters; coefficient C = 0.5A + B; coefficient D is a penalty factor that depends on the equivalent Poisson’s ratio. If the material is close to being incompressible, the value of I3 tends toward 1, and the penalty factor, D, approaches infinity. In our model, the quasi-incompressibility was obtained using Poisson’s ratio 0.49. Coefficients A and B were set to 1 and 5 GPa, respectively.
The fetal head was modeled as a rigid body formed by 8,010 shell elements. Its trajectory was imposed so to move as close as possible to the pubic rami to resemble the fetal head movement along the curve of Carus during vaginal delivery. Sliding contact was defined as being between the fetal head and the soft tissue. Soft-tissue boundary conditions were set with respect to the anatomy. The inner area behind the pubic rami had fixed displacements to simulate tissue connection to the bone. To fix the model in space, its outer edge was fixed for all degrees of freedom. In hands-on models, finger movement was simulated by the imposed movement of nodes corresponding to the area of the fingers in contact with the skin. In model B, the movement was imposed in two directions and in model C in the posterior direction only. Other degrees of freedom of the area of the fingers remained free.
Testing MPP on the model
Vaginal delivery of the fetal head was simulated using the model. The modified Viennese method described previously [14] was used to simulate the hands-on MPP technique. The area of contact between the fingers of the right-posterior hand and the perineal skin was calculated from the experimental measurements of finger imprints. The area covered by the accoucheur’s thumb and index finger corresponded to 2.5 cm2 and 2 cm2, respectively. We calculated the exact timing and location of finger application to the perineum and a coordinated movement between thumb and index finger using experimental stereophotogrammetric measurements from a pair of images taken in two different positions at the same time. It was calculated that fingers were applied when the vaginal introitus was dilated to 8 cm anteroposteriorly and 4 cm transversely. In the model, the fingers were applied when the anteroposterior diameter was 7 cm and the transverse diameter was 5.3 cm.
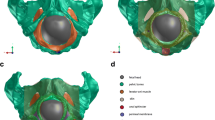
In model B, the thumb and index finger were placed alongside the fourchette and vaginal opening 11 cm apart and squeezed against the vector of the principal strain, 1 cm medially toward each other and 1 cm posteriorly toward the fourchette. Finger positions were not changed until expulsion of the fetal head was simulated (Fig. 1). In model C, a weaker grip between thumb and index finger was tested. The fingers were placed alongside the fourchette and vaginal opening 11 cm apart, together moved the touched skin 1 cm posteriorly toward the fourchette, but were not moved medially toward each other and remained 11 cm apart (Fig. 1). Axial and sagittal planes of the fetal head and perineal structures at the moment of fetal head expulsion, are shown with a color spectrum in multiples of stress units in Figs. 2 and 3, respectively.
Application and coordination of the thumb and index finger of the dominant-posterior hand. Model A: hands-off; model B: hands-on (squeezed 1 cm medially toward each other and 1 cm posteriorly toward the fourchette, fingertips remain 10 cm apart); model C: hands-on (squeezed 1 cm posteriorly toward the fourchette, fingertips remain 11 cm apart). Blue, soft tissue; green, fetal head; yellow dots finger location at the time of application (first line) and final location (second line)
Axial planes of the perineum and stress distribution in tissue, with a color spectrum in multiples of stress units at the moment of fetal-head expulsion. Model A hands-off; model B hands-on (squeezed 1 cm medially toward each other and 1 cm posteriorly toward the fourchette, fingertips remain 10 cm apart); model C hands-on (squeezed 1 cm posteriorly toward the fourchette, fingertips remain 11 cm apart). General view (first line), details of the perineum (second line)
Details of sagittal planes of the perineum and stress distribution in tissue, with a color spectrum in multiples of stress units at the moment of fetal-head expulsion. Model A hands-off; model B hands-on (squeezed 1 cm medially toward each other and 1 cm posteriorly toward the fourchette, fingertips remain 10 cm apart); model C hands-on (squeezed 1 cm posteriorly toward the fourchette, fingertips remain 11 cm apart)
To facilitate comparison, perineal tissue tension was calculated in stress units, with the maximum measured tension in the hands-off model at 100 % and at rest at 0 %. The following variables in all three models were evaluated: maximum perineal tension for each model, size of areas of the proportionate tension for each model (in divisions of 20 %, i.e., 0–20 %, 20–40 %, 40–60 %, 60–80 %, and 80–100 % of stress units) (Table 1), size of areas with aggregate proportionate tension for each model (in divisions of 20 %, i.e., ≥20 %, ≥40 %, ≥60 %, and ≥80 % of stress units) (Table 2), and maximum tension on the inner surface of the perineum at each 2-mm interval from the posterior fourchette (Table 3). No statistical analysis was performed due to the nature of the study.
Results
This study revealed that for the stress distribution with the hands-off technique, the highest tension in the midline at the time of fetal-head expulsion was at the fourchette. Stress distribution with hands-on MPP showed the same location of the maximum tension at the equivalent moment of fetal head expulsion. Table 3 shows that using MPP, stress peak decreased by 39 % in model B and 30 % in model C compared with the hands-off technique. Cross sections through the midline of the perineal body (Fig. 3) revealed that in the hands-off method, the area of tissue tension >20, >40, and >60 units of stress was significantly larger compared with the hands-on techniques (Tables 1 and 2). Table 2 shows that in the hands-off technique, nearly 30 % of the perineal area was exposed to tension ≥20 stress units, whereas in the hands-on technique used in model B, the exposed area of tension ≥20 stress units was only 10 % and in model C 15 %.
Discussion
The aim of this study was to assess whether MPP can reduce perineal tension during delivery in comparison with the hands-off technique. Simulating vaginal delivery on this biomechanical model showed that according to the principles of mechanics, appropriately performed MPP reduced the maximum tension in perineal structures by 39 %. This novel perineal model allowed us to assess the effect of MPP on strain and stress on perineal tissue during a simulated vaginal delivery. The simulation focused on the correct positioning and coordination of the thumb and index finger of the accoucheur’s dominant-posterior hand in order to reduce the maximum principal—transverse (tangential) strain. Reduced strain in the posterior perineum was notable, and these results may help identify which manual procedures may reduce perineal injuries in clinical practice.
Perineal injuries are associated with many factors, and protecting the perineum against tearing during vaginal delivery is probably a multifactorial issue as well. More research is needed to assess how the accoucheur’s nondominant left-anterior hand and the remaining part of the dominant right-posterior hand could be utilized. To categorize MPP into hands-on, hands-poised, or hands-off techniques only is insufficient; more detailed description of the function of the accoucheur’s hands is needed to define MPP in an exact and understandable way that is reproducible and comparable with other methods. Previous studies [11–13, 32, 33] did not find the hands-on technique to be beneficial in reducing perineal trauma. However, these studies could be criticized for an imprecise methodological concept because the exact execution of hands-on MPP was neither described nor controlled, and hence the results of these studies must be interpreted with caution. In some other studies, MPP was found to be a protective factor for anal sphincter tears [34–40]. Also, in two of them [34, 35], the exact performance of MPP employed is missing. In a retrospective study by Pirhonen et al. [36], MPP was the only obstetric variable that significantly differed between two countries with similar quality perinatal care and remarkably different rates of severe perineal trauma. In studies by Laine et al. [37–39], Hals et al. [40], and Stedenfeldt et al. [41], several obstetric interventions were modified, resulting in a radical reduction of anal sphincter tear rate in Norway. Therefore, the exact role of MPP alone was difficult to assess.
The main limitation of this study is the lack of data regarding the material parameters of the perineal tissue. Therefore, various parameters were tested and evaluated according to their realistic behavior during the simulation. The shape of the bulging perineum and the previous experimental data (dilation of the vaginal introitus or change in perineal body length) served for this evaluation. The authors are aware of the main weakness, and so the study approach was based on general biomechanical principles. Absolute stress values achieved during simulations may differ with use of different material parameters and thus were not presented. The main message of this simulation is that there was a significant decrease in perineal tension when an adequate modification of MPP is executed. Simulations with different tested material parameters corresponding to much softer tissue showed a very similar proportionate reduction for individual modifications of MPP. At the moment, due to the lack of available data, results of this study cannot be compared with other studies that evaluate the behavior of the levator plate because the anatomic layout of the levator muscle and type of levator deformation regarding maximum strain is different than that of the perineal body. Another limitation is that this simulation was not a clinical study. There has yet to be a study on whether reducing maximum perineal tension, as shown in this computational study, can lead to clinical reduction of adverse anatomical and functional perineal outcomes. However, for future clinical evaluation, study methodologies and MPP depiction and individual clinical performance must be markedly improved to achieve reliable and reproducible results.
Conclusion
In a biomechanical assessment with a finite element model of vaginal delivery, appropriate application of the thumb and the index finger of the accoucheur’s dominant-posterior hand to the surface of the perineum during the second stage of delivery significantly reduced tissue tension throughout the entire thickness of the perineum. Thus, this intervention might be beneficial in reducing the rate and/or degree of obstetric perineal trauma.
References
Minassian VA, Jazayeri A, Prien SD, Timmons RL, Stumbo K (2002) Randomized trial of lidocaine ointment versus placebo for the treatment of postpartum perineal pain. Obstet Gynecol 100:1239–1243
Johnson A, Thakar R, Sultan AH (2012) Obstetric perineal wound infection: is there underreporting? Br J Nurs 21:S28, S30, S32-5
Connolly AM, Thorp JM Jr (1999) Childbirth-related perineal trauma; Clinical significance and prevention. Clin Obstet Gynecol 42:820–835
Raisanen S, Vehvilainen-Julkunen K, Gissler M, Heinonen S (2011) A population-based register study to determine indications for episiotomy in Finland. Int J Gynaecol Obstet 115:26–30
DeWees WB (1889) Relaxation and management of the perineum during parturition. JAMA 24:841–848
Ritgen G (1855) Ueber sein Dammschutzverfahren. Monatschrift fur Geburtskunde u Frauenkrankh 6:321–347
Berghella V, Baxter JK, Chauhan SP (2008) Evidence-based labor and delivery management. Am J Obstet Gynecol 199:445–454
National Institute for Health and Clinical Excellence Intrapartum care: care of healthy women and their babies during childbirth. RCOG 2007; CG55 London. www.nice.org.uk/CG55
Munro J, Jokinen M. Midwifery practice guideline: care of the perineum (2008) RCM evidence based guidelines for midwifery-led care in labour. 4th edn. Royal College of Midwives, London. www.rcm.org.uk
Trochez R, Waterfield M, Freeman RM (2011) Hands on or hands off the perineum: a survey of care of the perineum in labour (HOOPS). Int Urogynecol J 22:1279–1285
McCandlish R, Bowler U, van Asten H, Berridge G, Winter C, Sames L et al (1998) A randomised controlled trial of care of the perineum during second stage of normal labour. Br J Obstet Gynaecol 105:1262–1272
Mayerhofer K, Bodner-Adler B, Bodner K, Rabl M, Kaider A, Wagenbichler P et al (2002) Traditional care of the perineum during birth. A prospective, randomized, multicenter study of 1,076 women. J Reprod Med 47:477–482
Albers LL, Sedler KD, Bedrick EJ, Teaf D, Peralta P (2005) Midwifery care measures in the second stage of labor and reduction of genital tract trauma at birth: a randomized trial. J Midwifery Wom Health 50:365–372
Zemčík R, Karbanova J, Kalis V, Lobovsky L, Jansova J, Rusavy Z (2012) Stereophotogrammetry of the perineum during vaginal delivery. Int J Gynaecol Obstet 109:136–139
Tsai PJ, Oyama IA, Hiraoka M, Minaglia S, Thomas J, Kaneshiro B (2012) Perineal body length among different racial groups in the first stage of labor. Female Pelvic Med Reconstr Surg 18:165–167
Dua A, Whitworth M, Dugdale A, Hill S (2009) Perineal length: norms in gravid women in the first stage of labour. Int Urogynecol J Pelvic Floor Dysfunct 20:1361–1364
Kalis V, Karbanova J, Bukacova Z, Bednarova B, Rokyta Z, Kralickova M (2010) Anal dilation during labor. Int J Gynaecol Obstet 109:136–139
Reginelli A, Mandato Y, Cavaliere C, Pizza NL, Russo A, Cappabianca S et al (2012) Three-dimensional anal endosonography in depicting anal-canal anatomy. Radiol Med 117:759–771
Knowles AM, Knowles CH, Scott SM, Lunniss PJ (2008) Effects of age and gender on three-dimensional endoanal ultrasonography measurements: development of normal ranges. Tech Coloproctol 12:323–329
Handa VL, Lockhart ME, Fielding JR, Bradley CS, Brubaker L, Cundiff GW et al (2008) Racial differences in pelvic anatomy by magnetic resonance imaging. Obstet Gynecol 111:914–920
Gupta S (2011) A comprehensive textbook of obstetrics and gynecology, Sec 1, 1st edn. Basic science in obstetrics and gynecology. Pelvic skeleton. Jaypee Brotherspp 23–30
Berger MB, Doumouchtsis SK, Delancey JO (2013) Bony pelvis dimensions in women with and without stress urinary incontinence. Neurourol Urodyn 32(1):37–42. doi:10.1002/nau.22275
Dietz HP, Shek C, Clarke B (2005) Biometry of the pubovisceral muscle and levator hiatus by three-dimensional pelvic floor ultrasound. Ultrasound Obstet Gynecol 25:580–585
Gregory WT, Nardos R, Worstell T, Thurmond A (2011) Measuring the levator hiatus with axial MRI sequences: adjusting the angle of acquisition. Neurourol Urodyn 30:113–116
Svabik K, Shek KL, Dietz HP (2009) How much does the levator hiatus have to stretch during childbirth? BJOG 116:1657–1662
Lien KC, DeLancey JO, Ashton-Miller JA (2009) Biomechanical analyses of the efficacy of patterns of maternal effort on second-stage progress. Obstet Gynecol 113:873–880
Schimpf MO, Harvie HS, Omotosho TB, Epstein LB, Jean-Michel M, Olivera CK et al (2010) Does vaginal size impact sexual activity and function? Int Urogynecol J 21:447–452
Ashton-Miller JA, Delancey JO (2009) On the biomechanics of vaginal birth and common sequelae. Annu Rev Biomed Eng 11:163–176
Altair, Hypermesh, Version 2012
ESI Group, Pamcrash, Version 2012
Fung YC (1993) Biomechanics—Mechanical properties of living tissues, 2nd edn. Springer-Verlag, New York
Foroughipour A, Firuzeh F, Ghahiri A, NorbaKhsh V, Heidari T (2011) The effect of perineal control with hands-on and hand-poised methods on perineal trauma and delivery outcome. J Res Med Sci 16:1040–1046
Sohrabi M, Bagha IR, Shirinkam R, Koushavar H (2009) A comparison of “hands off”versus “hands on”(Ritgen) techniques on perineal trauma during birth in nulliparous women. JAUMS 9:235–241
Parnell C, Langhoff-Roos J, Møller H (2001) Conduct of labor and rupture of the sphincter ani. Acta Obstet Gynecol Scand 80:256–261
Samuelsson E, Ladfors L, Wennerholm UB, Gareberg B, Nyberg K, Hagberg H (2000) Anal sphincter tears: prospective study of obstetric risk factors. BJOG 107:926–931
Pirhonen JP, Grenman SE, Haadem K, Gudmundsson S, Lindqvist P, Siihola S et al (1998) Frequency of anal sphincter rupture at delivery in Sweden and Finland–result of difference in manual help to the baby’s head. Acta Obstet Gynecol Scand 77:974–977
Laine K, Pirhonen T, Rolland R, Pirhonen J (2008) Decreasing the incidence of anal sphincter tears during delivery. Obstet Gynecol 111:1053–1057
Laine K, Skjeldestad FE, Sandvik L, Staff AC (2012) Incidence of obstetric anal sphincter injuries after training to protect the perineum: cohort study. BMJ. doi:10.1136/bmjopen-2012-001649
Laine K, Rotvold W, Staff AC (2013) Are obstetric anal sphincter ruptures preventable?- Large and consistent rupture rate variations between the Nordic countries and between delivery units in Norway. Acta Obstet Gynecol Scand 92:94–100
Hals E, Øian P, Pirhonen T, Gissler M, Hjelle S, Nilsen EB et al (2010) A multicenter interventional program to reduce the incidence of anal sphincter tears. Obstet Gynecol 116:901–908
Stedenfeldt M, Øian P, Gissler M, Blix E, Pirhonen J (2012) Risk factors for obstetric anal sphincter injury after a successful multicenter intervention programme. BJOG 119:724–730
Acknowledgments
The study was supported by the internal grant project SGS-2013-026 of the University of West Bohemia, by the European Regional Development Fund (ERDF), project “NTIS - New Technologies for the Information Society”, European Centre of Excellence, CZ.1.05/1.1.00/02.0090 and by the Charles University Research Fund (project number P36).
Ethical approval and funding
No formal ethical approval was required for this study; no external funding was obtained.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jansova, M., Kalis, V., Rusavy, Z. et al. Modeling manual perineal protection during vaginal delivery. Int Urogynecol J 25, 65–71 (2014). https://doi.org/10.1007/s00192-013-2164-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-013-2164-1