Abstract
Introduction
We set out to determine if insertion of a retropubic tension-free vaginal tape (TVT) sling at the time of pelvic organ prolapse surgery improves continence outcomes in women with pre-operative occult stress incontinence (OSI) or asymptomatic urodynamic stress incontinence (USI).
Methods
We conducted a randomised controlled study of prolapse surgery with or without a TVT midurethral sling. The pre- and post-operative assessment at 6 months included history, physical examination and urodynamic testing. Quality of life (QOL) and treatment success was assessed with the UDI-6 SF, IIQ-7 SF and a numerical success score. The primary outcome was symptomatic stress urinary incontinence (SUI) requiring continence surgery (TVT) at 6 months. Long-term follow-up continued to a minimum of 24 months. Secondary outcomes were quality of life parameters.
Results
Eighty women received prolapse surgery alone (n = 43) or prolapse surgery with concurrent TVT (n = 37). Six months following prolapse surgery 3 out of 43 (7 %) patients in the no TVT group requested sling surgery compared with 0 out of 37 (0 %) in the TVT group (ARR 7 % [95 %CI: 3 to 19 %], p = 0.11). After 24 months there was one further participant in the no TVT group who received a TVT for treatment of SUI compared with none in the TVT group (4 out of 43, 9.3 % versus 0 out of 37; ARR 9.3 % [95 %CI: −1 to 22 %], p = 0.06). Both groups showed improvement in QOL difference scores for within-group analysis, without difference between groups.
Conclusion
These results support a policy that routine insertion of a sling in women with OSI at the time of prolapse repair is questionable and should be subject to shared decision-making between clinician and patient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress urinary incontinence (SUI) often coexists with pelvic organ prolapse (POP). However, up to 80 % of women with POP do not complain of urinary incontinence. This is despite clinical and/or urodynamic testing revealing leakage of urine with or without reduction of the prolapse [1–5]. This phenomenon is described as occult stress incontinence (OSI). It is believed that urethral kinking due to bladder base descent prevents leakage of urine [2, 6, 7]. Reduction of the prolapse leads to a correction of the kinking, a decrease in urethral closure pressure and/or a decreased pressure transmission ratio unmasking SUI [6, 8, 9]. Successful prolapse surgery performed by the vaginal or abdominal route may be disappointing for the patient owing to the postoperative development of new onset incontinence.
The diagnosis of OSI has recently been defined as the presence of SUI on examination, or urodynamic stress incontinence in women with pelvic organ prolapse with a reduction of prolapse, who have no symptoms of SUI [10]. The prevalence of OSI will vary depending on how the condition is defined and how the patient is examined. In the Colpopexy and Urinary Reduction Efforts (CARE) study, patients were examined with a bladder volume of 300 ml. The incidence of OSI varied with the method used for prolapse reduction: pessary 6 %, manual 16 %, forceps 21 %, swab 20 % and speculum 30 % [11]. Women with OSI appear more prone to developing symptomatic postoperative SUI. Several studies have addressed the question of combining an anti-incontinence procedure with POP surgery either by vaginal, abdominal or laparoscopic routes after a diagnosis of OSI [9, 12–14]. The results of these studies show less postoperative SUI in women who have had the anti-incontinence procedure; however, the morbidity of concomitant SUI surgery, the overall patient benefit and the cost-effectiveness are still debated.
The aim of our study is to compare the outcomes of surgery for prolapse with and without the TVT retropubic sling in women diagnosed with OSI prior to surgery.
Materials and methods
A multicentre randomised controlled study was conducted after approval by the human ethics and research committee of the two participating hospitals (Mercy Hospital for Women, Monash Medical Centre). All methods and definitions conformed to standards recommended by the ICS and IUGA [10] except where specifically noted. Occult stress incontinence was defined in this study as stress urinary incontinence occurring and visualised in symptomatically stress-continent women either with or without prolapse reduction during urodynamic assessment. All women who needed surgical correction of POP routinely underwent urodynamic testing as part of their preoperative assessment. Only those women who had occult stress incontinence were invited to participate. Written informed consent was obtained.
Inclusion criteria for participation were pelvic organ prolapse greater than or equal to stage 2 requiring surgical correction, the absence of stress urinary leakage on history and demonstration of OSI utilising urodynamic assessment. Exclusion criteria included contraindications to pelvic surgery such as a pelvic infection, fistula, congenital or neurogenic bladder disorder, malignancy, or being medically unfit.
The pre- and post-operative protocol consisted of a comprehensive urogynecological history, physical examination, and multi-channel urodynamic testing. This included uroflowmetry (free flow study at the start and pressure flow study at the end of testing), resting urethral pressure profilometry before and after cystometry to a capacity up to 500 ml if tolerated. An estimation of Valsalva and cough leak point pressure in the semi-recumbent position with and without reduction of the prolapse and in the standing position without reduction of prolapse by direct visualisation of urinary incontinence was performed. A voiding pressure study completed the assessment. Prolapse reduction was performed in a semi-recumbent position utilising a Sims speculum or opened sponge forceps avoiding direct support of the urethra/anterior vaginal wall. The severity of prolapse was evaluated using the standard terminology of Pelvic Organ Prolapse Quantification (POPQ) recommended by the ICS [9].
The short form of the Urogenital Distress Inventory (UDI-6) and the Incontinence Impact Questionnaire (IIQ-7)) were used for subjective assessment of quality of life (QOL). Patients self evaluated the success of their procedure with a numerical success score ranging from 0 to 100 (0 corresponding with complete failure and 100 to complete cure).
Randomisation, was performed by the research nurse, in fixed blocks of 10 using a software package (Ranlist, University of Houston, Texas, USA 1996). Women were allocated to receiving TVT or not as part of their surgical treatment for pelvic organ prolapse. The surgeon was not involved in the randomisation and was informed about the allocation at the time of the surgery. The decision regarding the most suitable operation for prolapse treatment was not influenced by participation in this study. Prolapse repair was performed according to surgeon and patient preference. Participants were not blinded regarding their group allocation.
Regional or general anaesthesia was used accordingly after anaesthetic assessment. Prophylactic antibiotics were given at commencement of surgery. The TVT procedure (Gynecare; Ethicon, Somerville, NJ, USA) was performed as previously described by Ulmsten et al. [15–17]. Cystoscopy was used routinely to verify the absence of ureteric, bladder and urethral injury after all procedures (TVT and no TVT). Sling tensioning was achieved by a tension-free placement of fine dissecting scissors between the urethra and the tape without the aid of a cough test.
Postoperative catheter management depended on the duration of the vaginal pack (24 to 48 h postoperatively). A successful trial of voiding was defined by two post-void residual urine volumes <150 ml on ultrasound after removal of the pack and catheter. Short-term urinary retention or voiding difficulty was managed by reinsertion of an indwelling catheter for drainage and if persisting, the use of clean intermittent catheterisation until post-void residuals were satisfactory.
All women were reviewed at 6 weeks, 6 and 12 months following surgery and annually thereafter. At 6 months, urodynamic assessment, QOL questionnaires and numerical success score were completed. Yearly follow-up continues with clinical examination, quality of life questionnaires and numerical success scores. Neither participants nor assessors were blinded to group allocation. Participants would be reviewed randomly by one doctor of the team in the participating hospitals, often not the surgeon who performed the procedure.
The primary endpoint was the need for subsequent anti-incontinence surgery due to symptomatic SUI after 6 months. This report also includes further follow-up to 24 months and beyond. The decision regarding sling surgery in symptomatic women was based on the participant’s wishes and repeat urodynamic assessment. A TVT (or repeat TVT) procedure was offered regardless of group allocation. Secondary outcomes were subjective cure rates, intra- and postoperative complications, voiding function, urgency and urge urinary incontinence (UUI) symptoms, and change in quality of life as assessed by the UDI-6 and IIQ-7. The patient also reported a numerical success score that rated the overall satisfaction with the prolapse repair in addition to continence status after surgery.
The sample size calculation was performed based upon a reduction from 50 % to 10 % (absolute risk reduction of 40 %) in SUI after prolapse repair in the TVT group being clinically important. At a power of 90 % and a significance level of 0.05, the sample size estimate was 31 patients per group. The demographic data were tabulated, but no hypothesis testing was used to compare demographic data groups. Data were presented as mean (SD), median [25th to 75th percentile] {minimum, maximum} depending upon distribution or count (%). The primary outcome, the time until the patient requested repeat surgery for stress incontinence, was presented graphically using the Kaplan–Meier survival curve with survivorship between treatment groups tested using the log-rank test. Absolute risk reduction (ARR), number needed to benefit (NNTB) and numbers needed to harm (NNTH) for the combined procedure compared with prolapse repair alone were derived at 24 months postoperatively [18]. The UDI-6 and IIQ-7 quality of life scores hypothesis testing was based on the postoperative – preoperative difference scores using the Wilcoxon sign rank test (WST) for within group change and the Wilcoxon rank sum test (WRST) for between-group analysis for continuous data. Count data used exact two-sided hypothesis testing for between-group comparisons where appropriate. Significance level was set at 0.05 and adjusted for multiple comparisons using Holm’s step down procedure [19]. Analysis was performed using Stata v11 statistical software (Stata, College Station, TX, USA, 2009) with StatXact v9 (Cytel Software Corporation, Cambridge, MA, USA, 2010) used to perform exact hypothesis testing.
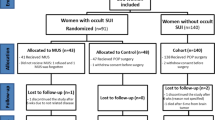
The trial was registered with the Australian New Zealand Clinical Trials Registry, ACTRN: 12611000844943. This study was reported according to the CONSORT checklist (Fig. 1).
Results
From June 2003 to August 2009 a total of 845 women with pelvic organ prolapse greater than or equal to stage 2 requiring surgical correction were screened and 146 women with occult stress incontinence were eligible. Therefore, the prevalence of occult stress incontinence using the study definition in our population was 17 % (95 %CI 15 % to 20 %). Eighty women who met the inclusion criteria and consented to participate were randomly assigned to prolapse surgery alone without a sling (n = 43) or prolapse surgery with concurrent TVT (n = 37). Sixty-six women declined participation because of personal preference regarding surgery or inconvenience of the trial commitments (Fig 1). All randomised participants received the allocated treatment. There was no difference in demographic characteristics such as age, parity, menopausal status, use of hormone replacement and prior continence or prolapse surgery between the two groups (Table 1). There was no difference in the number of participants with previous anterior vaginal repair as this could be considered a confounding factor between the two groups affecting continence status postoperatively. The type of prolapse surgery including anterior repair performed for participants was similar in the no TVT and TVT groups (Table 2).
The median follow-up time was 49.3 months (minimum 6 months to maximum 93.9). At 24 months 60 out of 77 patients (78 %) were still under observation. The overall completeness of follow-up (sum of active follow-up time for each patient/sum of potential follow-up time for each patient) was 69 %. The primary endpoint was the clinical need for stress incontinence surgery postoperatively. At 6 months following prolapse surgery 3 out of 43 patients in the no TVT group (7 %) requested sling surgery compared with 0 out of 37 (0 %) in the TVT group, ARR 7 % [95 %CI 3 % to 19 %], p = 0.11. After 24 months’ follow-up there was one further participant in the no TVT group who received a TVT for treatment of symptomatic SUI compared with none in the TVT group. The time from prolapse repair to sling insertion in the group of women with prolapse surgery alone was 1.8, 7.5, 9.3 and 27 months. The Kaplan–Meier survivorship curve of time to request for repeat surgery is presented in Fig. 2. There is some evidence of a difference in the proportion requiring repeat surgery between the prolapse alone and prolapse with TVT group (log-rank test p = 0.06). Assuming patients lost to follow-up did not require repeat surgery, the ARR at 24 months was 9.3 % [95 %CI −1.2 % to 22.2 %], p = 0.06 with NNTB 11 (95 %CI NNTH 83 to ∞, NNTB 5). If all patients lost to follow-up were treated as failures (no TVT =15 out of 43, TVT 6 out of 37) the ARR at 24 months was 18.7 % [95 %CI −1.3 % to 37.1 %], p = 0.06 with NNTB 6 (95 %CI NNTH 77 to ∞, NNTB 3).
There was no difference in intra- or postoperative complications between the two groups, in particular, those associated with insertion of a retropubic sling, such as bladder perforation, voiding difficulty (immediate with reinsertion of IDC for 24 h or clean intermittent self catheterisation for 6–10 days postoperatively) and haemorrhage (blood loss > 500 ml, no need for blood transfusion; see Table 2 for p values). No long-term voiding difficulty requiring catheterisation, loosening or division of sling were detected in either of the groups.
Urodynamic assessment was repeated 6 months following surgery in 60 of the participants (27 TVT and 33 no TVT). Twelve subjects (5 TVT and 7 no TVT) declined the repeat assessment as they were asymptomatic of stress urinary incontinence and declined further testing. There were eight participants (5 TVT [including 2 deceased participants] and 3 no TVT) who failed to attend the urodynamic assessment. Of the 60 women tested, in the TVT group 4 out of 27 (15 %) had USI demonstrated during repeat urodynamic assessment, compared with 22 out of 33 (66 %) in the no TVT group with an ARR of 52 % [95 %CI −71 % to −27 %, p < 0.001]. In these 26 women with USI 4 out of 4 (100 %) in the TVT group and 18 out of 22 (81 %) in the no TVT group reported no incontinence symptoms, ARR 18 % [95 %CI −40 % to 39 %, p = 0.40].
Based on the overactive bladder symptoms recorded preoperatively, there was no significant difference in the incidence of urgency (p = 0.47) and UUI p = 0.73 between groups. Cure rates for pre-existing urgency (TVT 20 out of 23, 87 % and no TVT 20 out of 23, 87 %) and UUI (TVT 13 out of 20, 65 % and no TVT 12 out of 23, 52 %) were similar in the two groups. The development of de novo urgency was also similar in the two groups (TVT 1 out of 6 ,17 % and no TVT 3 out of 13, 23 %) at 6 months compared with 2 out of 5 (40 %) and 0 out of 13 at 24 months. De novo UUI was more frequent in the TVT group than the no TVT group with 5 out of 10 (50 %) compared with 2 out of 13 (15 %) at 6 months and 5 out of 12 (42 %) compared with 2 out of 13 (15 %) at 24 months, but this did not reach statistical significance. The analysis of the treatment effect on overactive bladder symptoms is limited by the small numbers and some missing data.
The baseline QOL assessment (UDI-6, IIQ-7) of the two groups did not differ. Both groups showed improvement in QOL difference (postoperative – preoperative) scores for within group analysis (WST, p values < 0.001); however, no change was demonstrated between the 6- and 24-month assessments (WST, p values >0.1). There was no evidence of a difference in either QOL measure between the groups, adjusted for baseline score (WRST, p values > 0.2; Table 3). The numerical success score was high in both groups with no difference detected between the TVT and no TVT groups at 6 months (90 [95 % CI 80 to 100] vs 90 [95 % CI 80 to 95]) and 24 months (85 [95 % CI 70 to 95] vs 90 [95 % CI 80 to 98]) with Wilcoxon rank sum test p values >0.3). Combined success scores were 90 [95 % CI 80 to 100] and 90 [95 % CI 80 to 95] at 6 and 24 months respectively.
Discussion
There has been increased interest in the condition of occult stress incontinence and the most appropriate way to identify, counsel and treat women with this condition at the time of pelvic organ prolapse surgery. Two recent surveys in the UK and Australia/New Zealand showed that gynaecologists were evenly divided on whether they would routinely insert a midurethral sling in a women with symptomatic POP and OSI at the time of surgery [20, 21]. Anecdotally, there appears to be a large geographic variation in the practice of “prophylactic” sling insertion [22].
The results of this study question the value of routine insertion of a sling in women with OSI at the time of prolapse repair. If all participants lost to follow-up are assumed to be treatment successes or treatment failures, either 11 or 6 TVT slings would need to be inserted to prevent one patient requiring a TVT procedure within a median follow-up time of 49 months after prolapse correction.
Urodynamic stress incontinence persisted at 6 months in two thirds of women following prolapse surgery without TVT, although over 80 % were asymptomatic and did not require further surgical intervention during the follow-up period. It remains to be seen how many of these women, and of the 15 % of the TVT group who also had USI at 6 months, but were asymptomatic, will require continence surgery in the future.
Other studies have investigated the value of different anti-incontinence procedures such as needle suspensions, pubovaginal slings or fascial plication [9, 12–14] in women with POP and OSI.
Brubaker et al. compared abdominal sacrocolpopexy with and without Burch colposuspension in 302 women with POP and no symptoms of stress incontinence. Stress incontinence was present in women with POP and a concomitant Burch colposuspension in 23.8 % vs 44.1 % without Burch colposuspension at 3 months and in 32 % vs 45 % at 2 years respectively. The conclusion drawn from this study is that the addition of Burch colposupension to sacral colpopexy results in less stress urinary incontinence postoperatively [23, 24].
The Outcomes Following Vaginal Prolapse Repair and Midurethral Sling (OPUS) trial compared anterior vaginal prolapse repair with or without concurrent TVT sling procedure in stress continent women; one third of whom had OSI. At 12 months, urinary incontinence (positive stress test and/or bothersome urinary incontinence) was present in 45 out of 165 women (27 %) with a TVT and 74 out of 172 women (43 %) with no TVT. Complications of major haemorrhage and urinary retention were greater in the TVT group. The authors estimated that at 12 months 6.3 prophylactic slings would have to be inserted to prevent one woman from becoming stress incontinent after prolapse repair [25].
The assessment of overactive bladder symptoms did show a significant cure rate of pre-existing urinary urgency and UUI at 6 and 24 months in both groups with no statistical difference between the groups. This is most likely due to correction of the prolapse. The de novo occurrence of UUI was higher in the TVT group; however, statistical significance was not reached. This possible trend towards increased UUI with TVT is in agreement with other studies [26].
The numerical success scores and QOL questionnaires did not show any differences between the two groups. These scores indicate overall satisfaction with the surgery and may not be directly associated with sling insertion as the urinary incontinence was asymptomatic prior to the surgery and hence could not really be improved afterwards.
The strengths of this study include the randomised design, the length of follow-up that exceeds that of most other trials [25], minimisation of loss to follow-up and the use of validated outcome measures. The participants were identified through a stringent clinical screening process and urodynamic testing before prolapse correction. This allowed the inclusion of participants who satisfied the trial definition of OSI based on the information available at the start of the trial in 2003. We acknowledge that OSI is now defined as “stress incontinence on prolapse reduction” by the ICS/IUGA committee for standardisation of terminology in 2010 [10].
The limitations of this study include no treatment allocation concealment and no blinding of participants or assessors. Any future study design would benefit from a double-blinded design with sham dressings in the no treatment arm, although it is difficult to see how this would have an effect on the 6-month urodynamic findings or the 24-month long-term follow-up. It is likely that our study is underpowered to detect a difference in SUI between the two study groups. Our power calculation was based upon a 50 % incidence of SUI in the no TVT group; however, the actual incidence was found to be only 9.3 %. Given a true SUI rate of 10 % in a control group, a clinically important difference considered to be a 50 % absolute risk reduction (ARR) to 5 % in the TVT group would require a sample size of 620 per group to provide a power of 0.9.
Conclusion
The low number of sling procedures required in the non-intervention group to correct symptomatic stress urinary incontinence after more than 24 months’ follow-up supports the view that routine insertion of a sling in women with OSI at the time of prolapse repair is questionable and should be subject to shared decision-making. Women should be carefully counselled regarding the possibility of SUI occurring postoperatively and the risks as well as the benefits of concomitant sling surgery. These findings also have implications for the routine use of urodynamic assessment in the detection of OSI and would suggest that they might not be warranted for this purpose in the patient asymptomatic for SUI prior to prolapse surgery.
Further randomised studies including analyses of the health economics costing of the two approaches are needed to support either a prophylactic or a secondary procedure.
References
Gordon D, Groutz A, Wolman I, Lessing JB, David MP (1999) Development of postoperative urinary stress incontinence in clinically continent patients undergoing prophylactic Kelly plication during genitourinary prolapse repair. Neurourol Urodyn 18(3):193–197, discussion 197–198
Richardson DA, Bent AE, Ostergard DR (1983) The effect of uterovaginal prolapse on urethrovesical pressure dynamics. Am J Obstet Gynecol 146(8):901–905
Rosenzweig BA, Pushkin S, Blumenfeld D, Bhatia NN (1992) Prevalence of abnormal urodynamic test results in continent women with severe genitourinary prolapse. Obstet Gynecol 79(4):539–542
Veronikis DK, Nichols DH, Wakamatsu MM (1997) The incidence of low-pressure urethra as a function of prolapse-reducing technique in patients with massive pelvic organ prolapse (maximum descent at all vaginal sites). Am J Obstet Gynecol 177(6):1305–1313, discussion 1313–1314
Chaikin DC, Groutz A, Blaivas JG (2000) Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol 163(2):531–534
Bump RC, Fantl JA, Hurt WG (1988) The mechanism of urinary continence in women with severe uterovaginal prolapse: results of barrier studies. Obstet Gynecol 72(3 Pt 1):291–295
Borstad E, Rud T (1989) The risk of developing urinary stress-incontinence after vaginal repair in continent women. A clinical and urodynamic follow-up study. Acta Obstet Gynecol Scand 68(6):545–549
Bergman A, Koonings PP, Ballard CA (1988) Predicting postoperative urinary incontinence development in women undergoing operation for genitourinary prolapse. Am J Obstet Gynecol 158(5):1171–1175
Bump RC, Hurt WG, Theofrastous JP et al (1996) Randomized prospective comparison of needle colposuspension versus endopelvic fascia plication for potential stress incontinence prophylaxis in women undergoing vaginal reconstruction for stage III or IV pelvic organ prolapse. Am J Obstet Gynecol 175(2):326–333, discussion 333–335
Haylen BT, de Ridder D, Freeman RM et al (2010) An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J Pelvic Floor Dysfunct 21(1):5–26
Visco AG, Brubaker L, Nygaard I et al (2008) The role of preoperative urodynamic testing in stress-continent women undergoing sacrocolpopexy: the Colpopexy and Urinary Reduction Efforts (CARE) randomized surgical trial. Int Urogynecol J Pelvic Floor Dysfunct 19(5):607–614
Barnes NM, Dmochowski RR, Park R, Nitti VW (2002) Pubovaginal sling and pelvic prolapse repair in women with occult stress urinary incontinence: effect on postoperative emptying and voiding symptoms. Urology 59(6):856–860
Colombo M, Maggioni A, Scalambrino S, Vitobello D, Milani R (1997) Surgery for genitourinary prolapse and stress incontinence: a randomized trial of posterior pubourethral ligament plication and Pereyra suspension. Am J Obstet Gynecol 176(2):337–343
Groutz A, Gordon D, Wolman I et al (2000) The use of prophylactic Stamey bladder neck suspension to prevent post-operative stress urinary incontinence in clinically continent women undergoing genitourinary prolapse repair. Neurourol Urodyn 19(6):671–676
Ulmsten U, Falconer C, Johnson P et al (1998) A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 9(4):210–213
Nilsson CG (1998) The tensionfree vaginal tape procedure (TVT) for treatment of female urinary incontinence. A minimal invasive surgical procedure. Acta Obstet Gynecol Scand Suppl 168:34–37
Ulmsten U, Henriksson L, Johnson P, Varhos G (1996) An ambulatory surgical procedure under local anesthesia for treatment of female urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 7(2):81–85, discussion 85–86
Altman DG (1998) Confidence intervals for the number needed to treat. BMJ 317(7168):1309–1312
Ludbrook J (1998) Multiple comparison procedures updated. Clin Exp Pharmacol Physiol 25(12):1032–1037
Jha S, Moran PA (2007) National survey on the management of prolapse in the UK. Neurourol Urodyn 26(3):325–331, discussion 332
Vanspauwen R, Seman E, Dwyer P (2010) Survey of current management of prolapse in Australia and New Zealand. Aust N Z J Obstet Gynaecol 50(3):262–267
Fatton B (2009) Is there any evidence to advocate SUI prevention in continent women undergoing prolapse repair? An overview. Int Urogynecol J Pelvic Floor Dysfunct 20(2):235–245
Brubaker L, Cundiff GW, Fine P (2006) Abdominal sacrocolpopexy with Burch colposuspension to reduce urinary stress incontinence. N Engl J Med 354(15):1557–1566
Brubaker L, Nygaard I, Richter HE (2008) Two-year outcomes after sacrocolpopexy with and without burch to prevent stress urinary incontinence. Obstet Gynecol 112(1):49–55
Wei JT, Nygaard I, Richter HE et al (2012) A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med 366(25):2358–2367
Lee JK, Dwyer PL, Rosamilia A, Lim YN, Polyakov A, Stav K (2011) Persistence of urgency and urge urinary incontinence in women with mixed urinary symptoms after midurethral slings: a multivariate analysis. BJOG 118(7):798–805
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schierlitz, L., Dwyer, P.L., Rosamilia, A. et al. Pelvic organ prolapse surgery with and without tension-free vaginal tape in women with occult or asymptomatic urodynamic stress incontinence: a randomised controlled trial. Int Urogynecol J 25, 33–40 (2014). https://doi.org/10.1007/s00192-013-2150-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-013-2150-7