Abstract
Introduction and hypothesis
Our aim was to use three-dimensional enodovaginal ultrasound (3D EVUS) to identify sonographic parameters that are associated with successful outcomes following injection of Macroplastique.
Methods
Three hundred and sixty degree 3D EVUS was performed in 100 treatment-naïve patients following Macroplastique injection. The location, volumes, periurethral distribution, and distance of the hyperechoic densities from the urethrovesical junction were assessed. The patients were divided into two groups: group A (n = 72): patients who had good clinical outcome and group B (n = 28): patients who were not improved or worsened. The two groups were compared with respect to the ultrasound parameters measured.
Results
Group A had a greater proportion of women with Macroplastique located in the proximal urethra, while midurethral location was found to be significantly more frequent in group B (p = 0.036). The odds of a circumferential periurethral distribution in group A were 13.62 times the odds in group B (95% CI: 5.12–56.95). When the location of the injection and the type of periurethral distribution were considered together, it was found that when the site of injection was proximal, the odds of circumferential distribution in group A was significantly greater than those in group B (odds ratio [95% CI]: 22 [3.05–203.49]; p < 0.001).
Conclusion
Proximally located Macroplastique and circumferential periurethral distribution of Macroplastique are individually associated with successful outcomes following the injection. The combination of circumferentially distributed and proximally located Macroplastique is associated with the best short-term clinical outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transurethral injection of bulking agents is a viable alternative to surgery for patients with persistent or recurrent stress urinary incontinence (SUI) due to intrinsic sphincter deficiency. Various urethral bulking agents have been used in treating female stress urinary incontinence in the last decade. Macroplastique (Uroplasty, Minnetonka, MN, USA) is a silicone-based synthetic bulking agent that has been used in Europe since 1991, was introduced onto the US market in 2007 [1], and is gaining popularity in selected patient populations. It is composed of soft, flexible, highly textured polydimethylsiloxane (solid silicone elastomer) implants suspended in an inert, excretable water-soluble hydrogel [1]. Once injected, the implants agglomerate together, elicit a normal inflammatory response from the host, are encapsulated in collagen, and become anchored within the urethral tissue, remaining in place without moving or migrating [2]. It acts by increasing the urethral closure pressure and increasing resistance to urinary flow [1].
However, success rates are highly variable [3]. The optimal site for injection and the amount to be injected is still unclear. The decision to perform repeat injections is largely empirical and is generally based on patient reporting on the post-procedure impact on continence [4]. Thus, identifying the optimal site of injection and other intraoperative clinical parameters that can reliably predict outcomes following the injection is highly desirable and may improve the cost-effectiveness of the procedure.
Three-dimensional ultrasound imaging is an objective tool for the assessment of the lower urinary tract and pelvic floor. This new technique allows for more accurate and precise volume estimation than the conventional B-mode imaging, particularly for structures that are irregularly shaped [5]. Macroplastique (MPQ) can be clearly visualized in three-dimensional endovaginal ultrasound (3D EVUS) and therefore this technique may help to identify the optimal site for injection and the optimal periurethral distribution of the MPQ that are associated with improved outcomes.
The aim of this retrospective study was to identify three-dimensional sonographic parameters that are associated with successful outcomes following MPQ injection.
Materials and methods
Between April 2009 and January 2011, 100 treatment-naïve patients underwent 3D EVUS imaging before and immediately after their first MPQ injection in the Section of Urogynecology at the Cleveland Clinic Florida. All MPQ injections were performed by one of three fellowship-trained pelvic floor reconstructive surgeons. Three-dimensional endovaginal ultrasound was performed with BK Medical Ultrafocus (Peabody, MA, USA) by a fellow trained in ultrasound who was blinded to the site and number of injections. The post-procedure ultrasound was performed approximately 15 min following the injection with a bladder volume of approximately 150 cc. The ultrasound technique adopted is reproducible and has been found to have good inter-rater reliability for the assessment of the urethral complex [6]. 3D images were obtained with a 6-MHz 2052 transvaginal probe with 360° imaging capability. A length of 6 cm was scanned in 60 s with scans every 0.25 mm, thus obtaining 240 scans cumulatively, from which a three-dimensionally rendered cube was calculated. Each three-dimensional cube was digitally catalogued for future analysis.
After receiving Institutional Review Board approval, we retrospectively reviewed the clinical charts of the patients to obtain demographic information, medical and surgical history, clinical examination, and follow-up results. The definitions used in the study conform to the IUGA/ICS terminology for pelvic floor dysfunction [7].
All patients undergoing MPQ injection received pretreatment work-up of a detailed history, physical examination, and urinalysis. The Pelvic Organ Prolapse Quantification (POP-Q) system examination was performed prior to the procedure to determine the concomitant presence of prolapse. Multichannel urodynamic testing using Laborie XLT Aquarius urodynamic equipment (Laborie Medical Technologies, Toronto, Canada) was performed with air-charged catheters. Indications for MPQ injection included urodynamically diagnosed SUI with/without intrinsic sphincter deficiency (ISD). ISD was defined using the urodynamic diagnostic parameters of maximal urethral closure pressure (MUCP) of ≤20 cm H2O and/or Valsalva leak point pressure (VLLP) of ≤60 cm H2O. Operative reports were reviewed to obtain information regarding amount of MPQ injected, sites of injection, and complications, if any.
On follow-up, treatment success was determined based on subjective patient satisfaction and a self-reported incontinence severity scale. The incontinence severity scale included: 0: continence, 1: 1–2 incontinent events/day; 2: 3–4 incontinent events/day; 3: ≥ 5 incontinent events/day).
Treatment success was defined as a decrease of at least 1 in the incontinence severity score in addition to subjective improvement or cure. Treatment failure was either no change/an increase in the incontinence severity score or a subjective patient assessment of no improvement/worsening of symptoms.
The procedures were all carried out on an office basis. Injections were performed using an operating cystoscope (straight eyepiece with a 0 degrees angle of view) and an injection gun and a rigid hollow needle. The needle was inserted through the operating channel and visualized within the bladder. The cystoscope was withdrawn to proximal or midurethra and the urethral wall punctured at an angle of 45°. After insertion to a depth of 0.5 cm, the needle was advanced parallel to the urethral lumen for a further 0.5 cm. Using the injection gun, approximately 1–2.5 ml of MPQ was injected at each site. The needle was then withdrawn into the operating channel after approximately 30 s. The procedure was performed most often at two sites (3 o’ clock and 9 o’ clock positions) around the urethra and less frequently at only one or three sites. Injection was continued until complete coaptation of the urethral mucosa was seen. Care was taken to avoid passing the cystoscope through the injection sites to leave the MPQ blebs undisturbed. A voiding trial was performed following the procedure. The patients were instructed to void every 2 h for the next 48 h and were scheduled for the first follow-up visit after 2 weeks. Subsequent follow-up visits were scheduled at 6 weeks, 3 months, 6 months, and 12 months following the procedure.
The stored 3D cubes of the 100 patients were retrospectively reviewed. The injected MPQ could be seen as hyperechoic densities around the urethra. The periurethral distribution, location, volume of the hyperechoic densities and the distance of the injected material from the urethrovesical junction were assessed.
Assessment of periurethral distribution
The circumference of the urethra was divided into four quadrants in a clockwise fashion in the axial plane: left upper quadrant (12 to 3 o’ clock position); left lower quadrant (3 to 6 o’clock position); right lower quadrant (6 to 9 o’clock position), and right upper quadrant (9 to 12 o’clock position). The area of MPQ in each quadrant was determined.
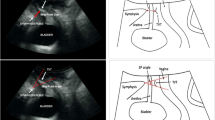
The 3D cube was manipulated to determine the axial plane in which the instillation of MPQ was maximal. The area of each quadrant filled with MPQ was determined in the selected axial plane. Each quadrant was considered to be adequately filled if more than 50% of the area of the quadrant in the selected axial plane was filled with MPQ. The 3D cube for each patient was then assessed to determine the number of quadrants adequately filled with MPQ: if more than 50% of the area of three consecutive quadrants or all four quadrants were filled with MPQ, the patient was considered to have “circumferential” distribution (Fig. 1). If less than 50% of the area of three consecutive quadrants or only two or one quadrants were filled with MPQ, the patient was considered to have “partial” distribution (Fig. 2).
Assessment of the location of the injected MPQ
The urethra was also divided along its length into three equal sections in the sagittal plane: proximal, middle, and distal. The site of injection was considered to be the proximal urethra, the midurethra or both if more than 50% of the area of either or both was filled with MPQ instillation.
Assessment of volume and distance of the MPQ from the urethrovesical junction
The urethra was divided into left and right quadrants in the sagittal plane and the volume of the material in each quadrant was calculated from the measurement of the diameter of the instilled MPQ along three axes. The distance of the injected MPQ from the urethrovesical junction was determined by calculating the mean of the distance of the proximal limit of the left and right injected volumes from the urethrovesical junction. The patients were divided into two groups: patients who experienced good clinical outcome following the first MPQ injection (group A, n = 72) and patients who did not derive any benefit from MPQ (group B, n = 28). Specifically, group A included patients who reported a decrease of at least 1 in the incontinence severity score in addition to subjective improvement or cure on follow-up. Group B included patients who reported either no change/an increase in the incontinence severity score or a subjective assessment of no improvement/worsening of symptoms.
The two groups were then compared with respect to their demographic data, medical, urogynecological and surgical history, number of previous anti-incontinence procedures, hormonal status, and total amount of injected MPQ. The 3D EVUS scan results regarding the volume, location, periurethral distribution, and the distance of the injected material from the urethrovesical junction (obtained as described above) of the two groups were also compared to determine whether any particular distribution pattern was associated with clinical success.
Statistical analysis was performed using PASW STATISTICS 18 (SPSS, Chicago, IL, USA). Continuous variables were tested for normality using Shapiro–Wilk normality test. Normal continuous variables were analyzed using Student’s t test and non-normal data were analyzed using the Mann–Whitney U test. Categorical data were analyzed using Chi-squared test or Fisher’s exact test where applicable. Binomial logistic regression analysis was performed to determine whether the location and number of sites of injection can predict the type of periurethral distribution. A p value < 0.05 was considered significant.
Results
The injection of MPQ proved to be a safe procedure, without serious perioperative morbidity or complications. All 100 patients who underwent 3D EVUS before and immediately after the first MPQ injection had follow-up data available and their 3D cubes could be analyzed satisfactorily. The two groups were similar with respect to their demographic data on the age of the patients, body mass index (BMI), parity, smoking history, menopausal status, and surgical history (p > 0.05; Table 1).
The two groups were similar with respect to the number of previous anti-incontinence procedures (Table 1). The indications for the MPQ injection in the two groups were also similar and included patients with SUI and with/without ISD (Table 1).
The two groups matched with respect to the number of weeks of follow-up (median [interquartile range] of 19.32 [14.89] in group A and 17.77 [8.36] in group B; p = 0.861). Treatment parameters were as detailed in Table 2. The number of pretreatment incontinent episodes per day was also similar in the two groups (median [Interquartile range]: 3.7 [1.75] in group A and 3.5 [1.9] in group B; p = 0.43).
Outcomes
There was a significant reduction in the number of incontinent episodes per day post-injection compared with pre-injection in group A, but not in group B (median [interquartile range] post-injection: 1.5 [1.6] in group A [p < 0.001] and 3.8 [1.67] in group B [p = 0.052]). The median reduction in the number of incontinent episodes per day was significantly more in group A than in group B (median [interquartile range] of 1.75 [3.5] in group A compared with 0.00 [1.50] in group B; p = 0.02). Twenty-eight (39.2%) patients in group A complained of urgency following the procedure compared with 12 (42.86%) patients in group B (p = 0.113). Thus, a higher prevalence of urgency post-injection was not the reason for lack of subjective improvement in group B patients.
3D EVUS results
The two groups were similar with respect to their 3D EVUS parameters of volume of MPQ in each quadrant and distance of the MPQ from the urethrovesical junction (Table 3).
Overall, the injection was found to be proximally located in 49 patients, in the midurethra in 25 patients, and in both locations in 26 patients. When the location of the injection was compared in the two groups, (Table 3), the proportion of women with the injected material located proximally or in both positions (proximal and midurethra) was significantly greater in group A while the proportion of women with the MPQ located in the midurethra was significantly more in group B (p = 0.036).
The two groups differed significantly in the number of women who had a circumferential distribution compared with those with a partial distribution (Table 3). The odds of finding a circumferential distribution in group A was 13.62 times the odds in group B (95% CI: 5.12–56.95; p < 0.001).
When the location of the injection and the type of periurethral distribution were considered together (Fig. 3), it is evident that when the injected material was in the proximal urethra or both locations (proximal and midurethra), the odds of circumferential distribution in group A were significantly greater than those in group B (odds ratio [95% CI]: 22 [3.06–203.49; p < 0.001] for a proximal location and the odds ratio [95% CI]: 11.33 [1.02–172.67; p = 0.028] for both locations). However, when the injection was located in the midurethra, although the odds of circumferential distribution in group A were greater than those in group B, it was not statistically significant (OR: 6.750; 95 % CI: 0.88–61.247; p = 0.047).
Logistic regression analysis was performed to determine whether the location of injection (proximal, midurethral or both locations) and the number of sites of injection (2, 1 or 3 sites of injection) could predict the type of periurethral distribution. A test of the full model against a constant only model was not statistically significant, indicating that the two independent variables as a set did not reliably distinguish between circumferential and partial distribution (Chi-squared = 5.195, p = 0.268 with df = 4). Nagelkerke’s R2 of 0.07 indicated a poor relationship between prediction and grouping. Prediction success overall was only 67% (81.8% for circumferential distribution and 38.2% for partial distribution). The Wald criterion demonstrated that neither the location of the injection nor the number of sites of injection made a significant contribution to prediction (p > 0.05).
Discussion
Injection of urethral bulking agents has several advantages: it is a minimally invasive procedure performed under local anesthesia and is amenable to office therapy [8]. The newer synthetic bulking agents like MPQ (polydimethylsiloxane elastomer) improve upon early injection treatment options like collagen, autologous fat, and polytetrafluoroethylene, which were associated with the problems of resorption, allergic reaction or hypersensitivity and migration [2]. A multicenter randomized controlled trial has shown that at 12 months after treatment, 61.5% of patients who received MPQ had improved Stamey grade 1 compared with 48% of patients who received Contigen injections, and that the dry/cure rate was 36.9% in the MPQ group compared with 24.8% in the Contigen group (p < 0.05) [2]. Gumus et al. prospectively showed a statistically significant improvement in the quality of life at a median follow-up time of 58 months post MPQ injection [9].
However, the success rates reported in the literature for MPQ injections are highly variable. A review [3] of eight long-term trials (N = 507) showed that cure rates at 12 months post-treatment ranged between 20% and 71%, with improvement rates between 19% and 48% [2, 10–16]. With follow-up extending up to 60 months, cure rates reported have ranged from 18% to 40% and improvement rates have ranged between 33% and 39%, with repeated injections required to maintain efficacy [14, 16].
Despite dozens of publications, there remains no universally accepted or standardized injection method [17]. The optimal volume of material for injection during a single session, the ideal orientation of the injection needle, or the optimal number of reinjection sessions for any given agent (until clinical “failure” has been determined) have not been defined [18]. Transurethral injections are performed under cystoscopic control as it enables the bolus to be visualized and injection to be continued until adequate mucosal coaptation is achieved [19]. However, published data suggest that endoscopically confirmed coaptation does not necessarily correlate with long-term improvement in continence [20]. More crucially, the ideal injection site has yet to be determined [19]. Reported injection locations have included from the level of the midurethra all the way to the bladder neck [19]. A randomized controlled multisite trial of midurethral injection of Zuidex via the implacer versus proximal urethral injection of Contigen cystoscopically showed that the primary outcome measure of 50% reduction in urinary leakage on provocation was achieved in 84% of Contigen-treated women versus 65% of Zuidex-treated women [20]. However, the authors concluded that confounding multiple variables inherent in the study design made a detailed analysis of study outcomes difficult [21]. Currently, there is no evidence to suggest that either one of the sites usually used, proximal or midurethra, is superior to the other [19]. Therefore, there is a need to not only identify the ideal injection site, but also to develop certain intra-procedural parameters that can aid in optimizing outcomes, estimate whether repeat injections will be necessary, and appropriately counsel patients.
Ideal site for injection
The mechanism of continence in urethral injection therapy is uncertain; however, there are four postulated methods:
-
1.
Mucosal coaptation and improvement in the closure mechanism of the urethral sphincter in response to increased intra-abdominal pressure [22].
-
2.
Cephalad augmentation of the urethral length, which accounts for increased abdominal pressure transmission in the first quarter of the urethra [23].
-
3.
Prevention of bladder neck opening during stress [23].
-
4.
Reduction in urethral lumen size [24].
Placement of the injectable material in midurethra does not increase either the functional length of the urethra [23] or prevent bladder neck opening during episodes of stress [25]. It has therefore been suggested that bulking materials should be placed just distal to the urethrovesical junction and that the position of the injected material may be more important than its quantity for a good bulking effect [25, 26]. This is corroborated by our study. Table 3 shows that the number of patients with MPQ located in the proximal urethra or both the locations (proximal urethra and midurethra) was statistically greater in group A than in group B. The amount of MPQ injected in both groups, however, was similar (Table 2).
Previously, in a study of 23 women with transperineal ultrasound carried out before and after periurethral collagen injections, it was reported that short-term continence status was related to the height of the “collagen bumps” on either side of the bladder neck [26]. Continence was not achieved in the study if the “bumps” were located less than 10 mm from the bladder neck [26]. In another study of 31 women, in whom transperineal ultrasound was performed 3 months after the first periurethral collagen implant, a distance of collagen from the bladder neck of less than 7 mm was found to be associated with positive outcomes [27]. The threshold of 7 mm was found to have a sensitivity of 83.3%, specificity of 85.7%, positive predictive value of 93.7%, and negative predictive value of 66.6% [27]. Although these studies support instillation of the material in the proximal urethra, description of the implants in terms of the distance from the urethrovesical junction may not be adequate as it does not take into account the extent to which the proximal urethra is filled with the implant. For example, the implant may be only 3 mm in distance from the urethrovesical junction; however, it may only fill 10% of the proximal urethra and the rest of the implant may be placed mostly in the midurethra. In our study, we did not find any statistically significant difference in the distance of the MPQ implants from the urethrovesical junction between groups.
Periurethral distribution of MPQ
In our study, circumferential distribution of MPQ was found to be associated with significantly better clinical outcomes than partial distribution (Table 3; p < 0.001). Other studies have also commented on periurethral distribution of bulking agents and its correlation with clinical success. In a retrospective study of 46 women in whom 3D transperineal ultrasound was performed 4 to 12 weeks following the periurethral collagen injection, Defreitas et al. [4] found that a significantly greater proportion of women with a good clinical outcome had circumferentially distributed collagen on ultrasound (62%) compared with the women who did not benefit from the treatment (20%, p = 0.006). Conversely, a significantly greater proportion of women who did not benefit from the treatment had a partial distribution (68%) compared with the women with a good clinical outcome (29%; p = 0.0169). Radley et al. [12] performed transurethral 3D ultrasound in 9 patients after MPQ injection. They reported that in the 6 women with good outcome, echogenic MPQ foci were seen to almost completely encircle the urethra, whereas in the 3 women with persistent stress incontinence, urethral encirclement was incomplete, and large gaps were observed between echogenic areas [12].
However, in these two studies, the criteria used to define distributions were not based on actual area measurements that are replicable. In the paper by Defreitas et al., the term “asymmetric” was used to describe an ultrasound finding in which collagen was located in one area around the urethra predominantly; either right, left, anterior or posterior. Equal distribution of the collagen between the left and right sides of the urethra was termed “symmetric” and “circumferential” was used when the collagen was distributed in a circular or horseshoe configuration [4]. Our study provides standard criteria based on area cut-offs to define circumferential and partial distribution that can be reliably reproduced and used in both further studies and in practice. Poon et al. [28] reported that the volume of the injected material on ultrasound at which continence improvement was achieved following collagen injection spanned a fairly broad range, from 1 cm3 to more than 5 cm3. Thus, they argued that more than measuring the volume of the implant, 3D ultrasound assessment is necessary to determine how well the periurethral submucosal space is circumferentially “filled” in a given patient. Our study corroborates this fact: it was the distribution of injected material in the various quadrants considered together that was found to correlate with clinical outcomes. Volume measurements were not helpful as the same volume of injected material can often occupy two quadrants in one patient and three in another (Table 3).
Determination of periurethral distribution on three-dimensional endovaginal ultrasound following MPQ injection has several potential benefits. Our study suggests that circumferential periurethral distribution on ultrasound can be used as an intra-procedural parameter to predict short-term clinical outcomes. In a patient with partial distribution seen on ultrasound performed immediately after the injection, MPQ may be injected into an unfilled quadrant submucosally in the same visit so that circumferential distribution is obtained. Hence, repeat injections can be avoided or the number of repeat injections needed may be reduced, thus reducing patient bother and also the cumulative costs of the procedure. An ultrasound examination can also be performed during the follow-up visit in a patient with unsatisfactory improvement. The need for a repeat injection can be determined and the quadrants where the material needs to be injected could be mapped out. An ultrasound can be performed immediately after the repeat procedure to confirm the improved periurethral distribution of the MPQ. Although we did not follow up our patients in group B with repeat injections to convert the partial distribution into circumferential distribution, Defreitas et al. performed repeat collagen injections in 7 of their 27 patients who failed to improve after the first collagen treatment and converted the distribution from asymmetric to circumferential [4]. Of these 7 women, 6 had a good clinical response.
Our study provides clear evidence that injecting MPQ into the proximal urethra is associated with successful short-term outcomes. In centers with access to three-dimensional endovaginal ultrasound examination, circumferential distribution of the injection can be ensured in addition to confirming that the MPQ has been injected in a proximal location. 3D EVUS offers several advantages: in addition to providing a high-definition view of the pelvic floor structures that can be manipulated in any plane in which the tissue travels, it also offers a permanent record of anatomy for future review. Also, multi-compartment scanning can be done; namely, 180° anterior pelvic compartment scanning can be performed with an 8848 transducer [29] to confirm the findings of the 360° scan (Fig. 4). 3D transperineal ultrasound can also be used, in centers where it is available, to determine the symmetry of distribution of MPQ. In centers where 3D ultrasound is not available, 2D transperineal ultrasound can still be used post-procedure to determine whether the MPQ is circumferentially distributed, even though 2D US cannot give accurate periurethral distribution measurements.
The study has limitations. It is a retrospective study with a short median follow-up in both groups. However, the follow-up in many patients ended because they opted for a repeat injection. Forty-one patients in group A underwent a repeat injection as although they had clinical improvement, they wanted to ensure complete cure. Twenty-five patients in group B underwent repeat injection as the first injection had not led to any improvement. Another drawback is that we defined success based on subjective improvement and a patient-reported incontinence severity scale and not on urodynamic parameters. Also, our study only focused on treatment-naïve patients and studied ultrasound performed after only the first injection. However, our study has provided sufficient evidence to support the need for long-term prospective studies with a more rigid definition of success and longer follow-up, including patients undergoing repeat injections. The permanence and visibility of MPQ on ultrasound many months after injection would allow more long-term studies. To our knowledge, this is the first study to determine 3D ultrasound parameters associated with successful outcomes following MPQ injection.
Conclusion
In conclusion, three-dimensional ultrasound examination affords a simple way of accurately assessing the location and periurethral distribution of injected MPQ. Proximally located MPQ and circumferential periurethral distribution of MPQ are individually associated with successful outcomes following the injection. The combination of circumferentially distributed and proximally located MPQ is associated with best short-term clinical outcomes.
References
Ramsden M, Williams E, Siegel S (2010) Female stress urinary incontinence: office-based urethral bulking agent procedure. Urol Nurs 30(5):297–305
Ghoniem G, Corcos J, Comiter C, Bernhard P, Westney O, Herschorn S (2009) Cross-linked polydimethylsiloxane Injection for female stress urinary incontinence: Results of a multicenter, randomized, controlled, single-blind study. J Urol 181:204–210
Davila W (2011) Nonsurgical outpatient therapies for the management of female stress urinary incontinence: long-term effectiveness and durability. Adv Urol 2011:1–14
DeFreitas G, Wilson T, Zimmern P, Forte T (2003) Three dimensional ultrasonography: An objective outcome tool to assess collagen distribution in women with stress urinary incontinence. Urology 62:232–236
Athansiou S, Khullar V, Boos K, Salvatore S, Cardozo L (1999) Imaging of the urethral sphincter with three-dimensional ultrasound. Obstet Gynecol 94:295–301
Wieczoreck AP, Wozniak MM, Stankiewicz A, Santoro GA, Boqusiewicz M, Rechberger T (2012) 3-D high frequency endovaginal ultrasound of female urethral complex and assessment of inter-observer reliability. Eur J Radiol 81(1):e7–e12
Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, Monga A, Petri E, Rizk DE, Sand PK, Shaer GN (2010) An International Urogynecologic Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J 21:5–26
Lee H, Lee Y, Han J, Jeong J, Choo M, Lee K (2010) Transurethral injection of bulking agent for treatment of failed mid-urethral sling procedures. Int Urogynecol J 21:1479–1483
Gumus I, Kaygusuz I, Derbent A, Simavli S, Kafali H (2011) Effect of the macroplastique implantation system for stress incontinence in women with or without a history of an anti-incontinence operation. Int Urogynecol J 22:743–749
Koelbl H, Saz V, Doerfler D, Haeusler G, Sam C, Hanzal E (1998) Transurethral injection of silicone microimplants for intrinsic urethral sphincter deficiency. Obstet Gynecol 92(3):332–336
Maher C, O'Reilly B, Dwyer P, Carey M, Cornish A, Schluter P (2005) Pubovaginal sling versus transurethral Macroplastique for stress urinary incontinence and intrinsic sphincter deficiency: a prospective randomised controlled trial. BJOG 112(6):797–801
Radley S, Chapple C, Mitsogiannis I, Glass K (2001) Transurethral implantation of Macroplastique for the treatment of female stress urinary incontinence secondary to urethral sphincter deficiency. Eur Urol 39(4):383–389
Tamanini J, D'Ancona C, Tadini V, Netto N (2003) Macroplastique implantation system for the treatment of female stress urinary incontinence. J Urol 169(6):2229–2233
Tamanini J, D’Ancona C, Netto N (2006) Macroplastique implantation system for female stress urinary incontinence: long-term follow-up. J Endourol 20(12):1082–1086
ter Meulen PH, Berghmans LC, Nieman FH, van Kerrebroeck PE (2009) Effects of macroplastique implantation system for stress urinary incontinence and urethral hypermobility in women. Int Urogynecol J Pelvic Floor Dysfunc 20(2):177–183
Zullo M, Plotti F, Bellati F, Muzii L, Angioli R, Panici P (2005) Transurethral polydimethylsiloxane implantation: a valid option for the treatment of stress urinary incontinence due to intrinsic sphincter deficiency without urethral hypermobility. J Urol 173(3):898–902
Rovner E, Goudelocke C (2010) Which injectable to use in the treatment of intrinsic sphincter deficiency? Curr Opin Urol 20:296–301
Smith ARB, Daneshgari F, Dmochowski R et al (2005) Surgery for urinary incontinence in women. In: Abrams P, Cardozo L, Khoury S, Wein A (eds) Incontinence: 3rd International Consultation on Incontinence. Health Publications, Plymouth, UK, pp 1297–1370
Chapple C, Wein AJ, Brubaker L, Dmochowski R, Espuna PM, Haab F, Simon H (2005) Stress incontinence injection therapy: what is best for our patients? Eur Urol 48:552–565
Lightner D, Rovner E, Corcos J, Payne C, Brubaker L, Drutz H, Steinhoff G, Zuidex Study Group (2009) Randomized controlled multisite trial of injected bulking agents for women with intrinsic sphincter deficiency: mid-urethral injection of Zuidex via the Implacer versus proximal urethral injection of Contigen cystoscopically. Urology 74(4):771–775
Kim Y, Kattan M, Boone T (1997) Correlation of urodynamic results and urethral coaptation with success after transurethral collagen injection. Urology 50:941–948
Appell R (1994) Collagen injection therapy for urinary incontinence. Urol Clin North Am 21:177–182
Monga AK, Sl S (1997) Urodynamics: prediction, outcome and analysis of mechanism for cure of stress incontinence by periurethral collagen. Br J Obstet Gynaecol 104:158–162
Appell RA (1990) New developments: injectables for urethral incompetence in women. Int Urogynecol J 1:117–119
Benshushan A, Brzezinski A, Shoshani O, Rojansky N (1998) Periurethral injection for the treatment of urinary incontinence. Obstet Gynecol Surv 53:383–388
Khullar V, Cardozo LD, Abbott D, Hillard T, Norman S, Bourne T (1993) The mechanism of continence achieved with GAX collagen as determined by ultrasound (abstract). Neurourol Urodyn 78:439–440
Elia G, Bergman A (1996) Periurethral collagen implant: ultrasound assessment and prediction of outcome. Int Urogynecol J Pelvic Floor Dysfunct 7:335–338
Poon CI, Zimmern PE, Wilson TS, Defreitas GA, Foreman MR (2005) Three-dimensional ultrasonography to assess long-term durability of periurethral collagen in women with stress urinary incontinence due to intrinsic sphincter deficiency. Urology 65:60–64
Shobeiri SA, White D, Quiroz LH, Nihira MA (2012) Anterior and posterior compartment 3D endovaginal ultrasound anatomy based on direct histological comparison. Int Urogynecol J 23(8):1047–1053
Conflicts of interest
Aparna Hegde is funded by the IUGA International Fellowship Grant Award, 2011. G.W. Davila—consultant and honoraria: Astellas, Watson, American Medical System, Novasys Medical, CL Medical; research funding: American Medical Systems, Astellas. Other authors: no disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hegde, A., Smith, A.L., Aguilar, V.C. et al. Three-dimensional endovaginal ultrasound examination following injection of Macroplastique for stress urinary incontinence: outcomes based on location and periurethral distribution of the bulking agent. Int Urogynecol J 24, 1151–1159 (2013). https://doi.org/10.1007/s00192-012-1983-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-012-1983-9