Abstract
Introduction and hypothesis
We used the Valentini–Besson–Nelson (VBN) mathematical micturition model to analyze the potential obstructive effect of a 7-F transurethral catheter on the voiding process during intubated flow (IF) in women. Our hypothesis was that incomplete sphincter relaxation leads to residual sphincter pressure.
Methods
We reviewed a urodynamic database of women referred for evaluation of lower urinary tract dysfunction. Exclusion criteria were neurological disease or grade ≥2 prolapse. Eligible women underwent free uroflow (FF-1) before cystometry, an IF (7-F urethral catheter), and a second FF (FF-2) at the end of the session. Interpreted flows were restricted to voided volumes ≥100 ml and continuous flow patterns. Analysis of FF and IF was made using the VBN model.
Results
Among 472 women, 157 met the inclusion criteria. The effect of the urethral catheter was geometric only in 60 (38.2 %) patients. An additional effect, identified as incomplete sphincter relaxation, was observed in 97 (61.9 %) patients. Among this second group, the same residual sphincter excitation was found for 30 (30.97 %) patients during FF-2.
Conclusion
When comparing IF with FF with the VBN model, the decrease in maximum flow rate (Qmax) did not appear to result only from the geometric effect of the catheter but from incomplete sphincter relaxation during voiding, possibly because of patient’s anxiety or a urethral reflex induced by the presence of the catheter. These findings emphasize the need to perform an FF before the IF to strengthen the reliability of conclusions of a urodynamic investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is widely accepted that pressure-flow (PF) studies are the best method for assessing the voiding phase in micturition and that the transurethral catheter can have a potential irritant and/or obstructive effect. Both in men and in women, it is frequent to observe a large decrease in maximum flow rate (Qmax) during intubated flow (IF) compared with free uroflowmetry (FF) [1–6]. That observation implies that the urethral catheter may adversely affect uroflowmetry parameters, leading to an overestimation of bladder outlet obstruction (BOO). Previously published studies, mainly in men, attempted to investigate the obstructive effect of the urethral catheter during PF. However, results are controversial: significant obstructive effect of both 5-F and 10-F catheters in men with benign prostatic enlargement [1], and no significant effect of a 8-F catheter in men with lower urinary tract symptoms (LUTS) [7]. In women, data are more limited but are also controversial: significant obstructive effect of a 6-F [2] or 7-F catheter in healthy women [3], of different sized catheters [4, 5] or 7-F [6] in women with LUTS, positive effect of a 7-F catheter [8], and no effect of a 4-F catheter [9] or of two 5-F catheters [10]. The main cause of these discrepancies can be assigned to the impossibility of taking into account the volume and geometric effects of the catheter (i.e., partial urethral obstruction). Some authors thought that analyzing micturitions with similar voided volumes (varying < 20 %) on FF and IF could be the solution to the volume effect [4, 6, 11, 12]. They concluded to an obstructive effect due the presence of the catheter, but they could not distinguish between a geometric obstructive effect and dysfunctional voiding caused by either incomplete sphincter relaxation or fading detrusor excitation.
Modeling allows studying these hypotheses in greater depth. We made some attempts to explain the differences between data from FF and IF, and our conclusion was that urethral reflex leading to a compressive effect could be induced by the catheter in situ [13–15]. In this study, our purpose was to apply the Valentini–Besson–Nelson (VBN) mathematical micturition model [16] to successive uroflow recordings (FF, IF, and a second FF) of women referred for urodynamic evaluation of lower urinary tract dysfunction (LUTD) in order to analyze the potential effect of the urethral catheter on IF. Our hypothesis was that incomplete sphincter relaxation leads to residual sphincter pressure (RSP).
Materials and methods
Population and urodynamic study
This study was conducted in accordance with the Declaration of Helsinki. According to the local practice of our Ethics Committee, there is no formal Institutional Review Board approval required for retrospective studies. Urodynamic recordings of women who were referred for LUTD evaluation over a 2-year period (1 January 2008 to 31 December 2009) were reviewed. Exclusion criteria included history of neurological disease, diabetes mellitus, or grade ≥2 prolapse. All patients were evaluated using medical history, review of medications, bladder diary for at least 48 h including voiding times and voided volumes both day and night, physical examination, and dipstick urinalysis. Urodynamic sessions were performed using the Dorado® unit from Laborie. In our laboratory, each session included FF at arrival (FF-1), cystometry and intubated flow (IF), urethral pressure profilometry (UPP) with bladder refilled to 250 ml (or less in case of detrusor overactivity), and a second FF (FF-2). Cystometry was performed with the patient in the seated position with a 7-F triple-lumen urethral catheter perfused with saline at room temperature using a filling rate of 50 ml/min. Pressure transducers were zeroed to atmospheric pressure at the upper edge of the symphysis pubis. Rectal pressure was recorded using a punctured intrarectal balloon catheter filled with 2 ml of saline according to the report of Good Urodynamic Practice guidelines [17]. Maximum urethral closure pressure (MUCP) was obtained from UPP at rest in the supine position. Postvoid residual volumes (PVR) were measured by ultrasound (US) using a Bladder-Scan®.
VBN analysis
The VBN model is explained in Appendix 1. Analysis of urodynamic tracings (flow and pressure) using the VBN model allows evaluation of characteristic parameters, such as detrusor contractility (k), urethral parameter (compressive γ, or constrictive σ), and degree of nervous excitation of both detrusor and urethral sphincter. Effects of initial bladder volume and catheter size are taken into account in the model. The VBN model has a few stipulations before it can analyze a urodynamic tracing. First, analysis requires a Qmax > 2 ml/s, a voided volume ≥100 ml, and a continuous flow curve without predominant abdominal straining. A primary objective of this study was to analyze the three successive voidings performed during a urodynamic session. Granted, mechanical parameters (detrusor contractility and urethral parameter) do not change during a urodynamic session, but nervous excitation can differ between FF and IF. For the purpose of this analysis, it was assumed that complete sphincter relaxation occurred during FF-1, whereas IF and FF-2 were analyzed for RSP. Criteria to deem tracing acceptable for incorporation into our results were: (1) same value of mechanical parameters during the session, and (2) good fitting between recorded and computed curves, with a quadratic error <5 %.
Geometric obstruction due to the urethral catheter
As a catheter reduces the cross section of the urethra, this mechanical effect is taken into account as a constrictive effect by the VBN model. Hydrodynamic equations giving the flow rate vs. time are coupled with the law of urethral elasticity [16]: without catheter, the cross section of the urethra S(x), area of the fluid, is a function of local hydrodynamic pressure p(x). A urethral catheter (cross-section s) in situ does not modify the elasticity law S(p), but the area of the fluid becomes (S-s). A theoretical analysis of the geometric effect of the urethral catheter is provided in Appendix 2. Thus, a greater decrease in Qmax during IF must be analyzed as a nonmechanical effect of the catheter.
Study protocol
At the onset of voiding, the bladder has an initial volume (Vini). The resting sphincter pressure is taken as being equal to MUCP.
-
Step 1:
The first VBN analysis of IF, without hypothesis on RSP value (entries are ViniIF and catheter diameter), provides detrusor contractility k and a rough evaluation of what is termed a “whole urethral obstruction,” which includes catheter size, urethral parameter, and RSP effects together.
-
Step 2:
From FF-1, the VBN computation (entries are ViniFF-1 and k value; assumption RSP = 0) provides the value of the urethral parameter.
-
Step 3:
A second analysis of IF (entries are ViniIF, k, catheter diameter, and urethral parameter) then obtains an RSPIF value.
-
Step 4:
Analysis of FF-2 (entries are ViniFF-2, k, and urethral parameter) gives an independent value of RSP (RSPFF-2).
Theoretical study
A theoretical study was performed to analyze the sensitivity of flow parameters (Qmax and pdet.Qmax) to changes in main mechanical parameter value.
Statistical analysis
Data are presented as mean±standard deviation (SD) and range. The Wilcoxon signed rank test was used for comparison of related samples and analysis of variance (ANOVA) and the t test to compare unrelated samples. Statistical analysis was performed using SAS, version 5.0 (SAS Institute, Inc., Cary, NC, USA). All statistical results were considered significant at p < .05; the p value was adjusted in case of multiple comparisons.
Results
Among 472 files, 271 had available tracings for the three components: FF-1, IF and FF-2. However, because of the VBN requirements on Qmax, voided volume, and flow, the final analysis was conducted on a total of 157 files (Fig. 1). Among these 157 eligible women, mean age was 59.3 ± 15.5 (21–94) years; the main complaint was incontinence in 120 (32 stress, 45 mixed, 43 urge); other complaints were frequency in 16, dysuria in 16, and pelvic pain in five. We observed two groups that differed by the effect of the urethral catheter, the first in which there was only a geometric effect of the catheter, and the second in which an additional effect was noted. There were no significant differences in age and in main complaint.
Geometric effect only of urethral catheter
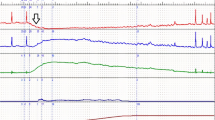
Figure 2) shows data of patients experiencing geometric effects only of the catheter.
Analysis of a case with only a mechanical effect of the catheter. Valentini–Besson–Nelson (VBN) parameters for initial free uroflow test (FF-1) and intubated flow (IF): detrusor force k = 1.3, urethral compressive parameter = 15 cm H2O. Left FF at arrival; right IF. The sphincter is completely relaxed during voiding. Q rec recorded flow, Q comp computed flow, p det.rec recorded detrusor pressure, p det.comp computed detrusor pressure, p sph.comp computed sphincter pressure
In 60/157 (38.2 %) files, QmaxFF-1 and QmaxIF were similar (Table 1). The same mechanical and nervous parameters allowed reproduction of FF-1 and IF. Among these files, 13 (21.7 %) had similar Vini.
Additional effect of urethral catheter
Figure 3 shows data for the group experiencing an additional effect from the catheter.
a, b Analysis of two cases with additional effect of the catheter in situ. The sphincter is incompletely relaxed during intubated flow (IF). As legends of the curves are identical to those in Fig. 2, and to make them easier to read, only the sphincter pressure curve is specified (continuous arrow). Note that for case b, an effective abdominal pressure (pabd.eff) (only acting on the bladder) must be added (dotted arrow) to restore the end of voiding, and that computed detrusor pressure (pdet.comp) + pabd.eff = recorded detrusor pressure (pdet.rec). a Valentini–Besson–Nelson (VBN) parameters: k = 0.25, normal urethra. The catheter effect does not persist for the second free uroflow (FF-2) study. b k = 0.5, normal urethra. The catheter effect during IF is a compression of 8 cm H2O, which persists for FF-2
In 97/157 (61.8 %) files, a large decrease in Qmax was observed during IF (QmaxFF-1 >> QmaxIF); 32 (32.9 %) had similar Vini. Flow and pressure curves during IF were restored to good fitting, assuming a urethral obstruction due to incomplete relaxation of the sphincter during voiding. RSP kept a constant value during voiding. The ratio RSP/MUCP was 0.39 ± 0.25. Among these 97 files, FF-2 showed no additional obstruction in 67 (69.1 %) (Fig. 3a), whereas similar RSP was observed during FF-2 and IF in the other 30 (29.9 %) (Fig. 3b). Note that PVR and voiding time increased significantly during IF, whatever the QmaxFF-1/QmaxIF ratio was, whereas pdet.Qmax was not significantly different (Table 1). Theoretical analysis demonstrated that the difference between groups resulted from urethral compression (Table 2).
Discussion
The presence of a urethral catheter during urodynamic studies can adversely affect flow curves, resulting at times in a polyphasic flow, low Qmax, and/or a prolonged flow time. Possible explanations for this effect include dysfunctional voiding due to anxiety or possibly an obstructive effect from the urethral catheter, but none can be easily verified. In fact, there is no consensus among findings of previous studies investigating the effect of a urethral catheter during urodynamic studies in women [2–10]. To try to sort out this dilemma, we apply the VBN model to urodynamic files to compare several noninvasive flow analysis (before and after cystometry) to an intubated flow. We were aided in this analysis by the originality of the VBN model, which allows analysis of the entire flow curve and not just the Qmax. The model was used to test the hypothesis that a urethral reflex mechanism induced by the catheter in situ provokes a remaining level of sphincter excitation.
Certainly, the VBN model takes into account the Vini, but unless great differences are noted during the voiding process (>500 ml or <200 ml), which is generally not the case, the usual volume variations do not affect the model (Appendix 2). The geometric effect of a catheter can be modeled as a constrictive obstruction [18]. Theoretical computations in women show that this effect is much too weak to explain the high differences observed between QmaxFF-1 and QmaxIF (Appendix 2). In our study, we observed similar Qmax for FF-1 and IF in 38 % of files. Therefore, could there be some element of abdominal straining during IF to explain that the Qmax during IF matched the Qmax during FF in these patients? As predominant abdominal straining is an exclusion criterion for VBN analysis, we do not feel that this is an acceptable explanation.
Thus, to explain the large decrease in Qmax during IF in 62 % of our files, we needed to search for an additional phenomenon. Two hypotheses can be proposed: a decrease of detrusor contractility or a urethral obstruction. The first is associated with a decrease of pdet.Qmax, the second with a slight increase of pdet.Qmax (due to the Hill–Griffiths law). The value of pdet.Qmax was not significantly different between the two groups. However the small increase observed in the group with lower QmaxIF is consistent with a urethral, not a detrusor, cause. The mechanic status of the bladder and urethra cannot change during a urodynamic session, but the nervous control can differ (without any pathological meaning) for successive voidings. So, we assumed the possibility of incomplete sphincter relaxation during voiding and concluded that the presence of a urethral catheter can at times evoke a urethral reflex mechanism. That residual sphincter excitation is a nerve-mediated phenomenon due to the presence of a foreign object in the urethra. This effect of the urethral catheter does not exclude other abnormalities, which can occur during the voiding process, such as a break in detrusor excitation [14] or abdominal straining. Note that if a break of detrusor excitation would also lead to decreased Qmax, then abdominal straining would have an inverse effect. In some cases, the obstructive effect induced by the catheter is observed during FF-2. A similar effect has been observed in rabbits: increased electrical activity of the external sphincter lasting for 14 min was demonstrated with introduction of a urethral catheter [19].
The first consequence of decreased Qmax is prolonged flow time. The second is increased PVR. These findings were once again noted in this study. This is consistent with reports in the existing literature. In particular, it has been shown [13] that voiding time in voidings with large PVR is inferior to the time needed for voids that ending with complete emptying. In addition, a previous study demonstrated that the end of voiding and return to continence occur 60 ± 20 s after flow onset [14] and result from concomitant sphincter closure and fading of detrusor excitation.
The limitations of this study include those linked to the VBN model, in that not all curves can be analyzed. Specifically, files with incomplete tracings or containing flows with voided volumes <100 ml could not be studied. Predominant abdominal straining is also excluded by the model. Although these limitations are worth detailing, it is established in real-life practice that flows from voided volume <150 ml can provide suspicious conclusions and that straining can alter the quality of the tracings, hence their interpretation. Furthermore, UPP is not always performed because it can affect urethral behavior during subsequent tests. In this study, instead of performing the UPP at the beginning, we were careful to perform cystometry and IF first, and then the UPP at the end of the urodynamic session. In this context, we could compare FFs before and after a significant degree of urethral manipulation, and in that sense enhance the potential for differential findings.
Conclusions
The large decrease in Qmax observed during IF when compared with FF during the same urodynamic session does not result only from the geometric effect of the catheter because it did not occur in 38 % of our analyzable files. In a large segment of our studied population, we noted a mechanism of incomplete sphincter relaxation during voiding, which we attributed to urethral reflex from the introduction of the urethral catheter. These findings underline the importance of obtaining FF before IF during a urodynamic session in order to increase the reliability of conclusions of the urodynamic investigation.
References
Klinger HC, Maderbasher S, Schmidbauer CP (1996) Impact of different sized catheters on pressure-flow studies in patients with benign prostatic hyperplasia. Neurourol Urodyn 15:473–481
Baseman AG, Baseman JJG, Zimmern PE, Lemack GE (2002) Effect of 6-F urethral catheterization on urinary flow rates during repeated pressure-flow studies in healthy female volunteers. Urology 59:843–846
Sorensen S, Janler M, Knudsen UB, Djuruus JC (1989) The influence of a urethral catheter and age on recorded urinary flow rates in healthy women. Scand J Urol Nephrol 23:261–266
Scaldazza CV, Morosetti C (2005) Effect of different sized transurethral catheters on pressure-flow studies in women with lower urinary tract symptoms. Urol Int 75:21–25
Costantini E, Mearini L, Biscotto S, Giannantoni A, Bini V, Porena M (2005) Impact of different sized catheters on pressure-flow studies in women with lower urinary tract symptoms. Neurourol Urodyn 24:106–110
Groutz A, Blaivas JG, Sassone AM (2000) Detrusor pressure uroflowmetry studies in women: effect of a 7Fr transurethral catheter. J Urol 164:109–114
Reynard JM, Lim C, Swami S, Abrams P (1996) The obstructive effect of a urethral catheter. J Urol 155:901–903
Haylen BT, Cerqui A, Law M, Dietz P (1999) Effect of a size 7Fr urethral catheter on urine flow rates in urogynaecology patients. Int Urogynecol J Suppl 10:S98, abstract Denver ICS-IUGA meeting, Informally discussed posters: Urodynamics
Di Grazia E, Bartolotta S, Salvia G et al (2002) Detrusor pressure uroflowmetry studies in women: effect of 4-Fr transurethral catheter. Arch Ital Urol Androl 74:134–137
Lose G, Thunedborg P, Jorgensen L, Colstrup H (1986) A comparison of spontaneous and intubated flow in female patients. Neurourol Urodyn 5:1–4
Richard P, Ordonez NI, Tu LM (2011) The effect of a 6Fr catheter on urodynamic studies: are they obstructive? AUA Annual meeting, 2011, May 14–19, abstract 2175
Gajanan SB, Girish GN, Chandrashekhar SR, Venkatesh GK (2011) Free uroflow versus pressure-flow urodynamic outcomes: does the transurethral catheter cause a measurement artifact? UroToday Int J. 2011 Jun 4(3). doi:10.3834/uij.1944-5784.2011.06.08
Valentini F, Marti B, Robain G, Nelson P (2008) Differences between the data from free flow and intubated flow in women with urinary incontinence. What do they mean? Neurourol Urodyn 27:297–300
Valentini FA, Mazières L, Nelson PP (2010) Can modeled analysis of urodynamic recordings help to demonstrate the nervous control of the bladder and urethra during micturition? UroToday Int J. Vol 3/Iss 4/August. doi:10.3834/iuj1944-5784.2010.08.10
Hennebelle D, Valentini F, Robain G, Nelson P (2011) Decrease of maximum flow rate during intubated flow is not only due to the urethral catheter in situ. AUA annual meeting, 2011, May 14–19, abstract 2176
Valentini FA, Besson GR, Nelson PP, Zimmern PE (2000) A mathematical micturition model to restore simple flow recordings in healthy and symptomatic individuals and enhance uroflow interpretation. Neurourol Urodyn 19:153–176
Schäfer W, Abrams P, Liao L et al (2002) Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn 21:261–274
Valentini FA, Besson GR, Nelson PP (1999) Effet obstructif d’un catheter urétral sur les parameters mictionnels: etude théorique. Prog Urol 9:361–370
Koraitim M (1982) Catheter as source of error in urodynamic study. Urology 20:223–225
Griffiths DJ (1980) Urodynamics: the mechanics and hydrodynamics of the lower urinary tract. Adam Hilger ed, Bristol
Griffiths DJ (1973) The mechanics of the urethra and of micturition. BJU 45:497–507
Besson G, Valentini F, Nelson P (1996) Progress in the theory of flow through the urethra during micturition. In International Continence Society 26th Annual meeting. Bologna: Monduzzi ed. pp 39–43
Acknowledgements
We thank Pr. Philippe Zimmern (The University of Texas, South-Western Medical Center, Dallas, TX, USA) who kindly agreed to discuss the manuscript
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
The Valentini–Besson–Nelson (VBN) mathematical model [16] is a quantitative description of the mechanistic phenomena governing micturition; these phenomena are bladder contractility, elasticity, and viscoelasticity [20]; urethral elasticity [21]; urethral compression by the sphincter; and turbulent incompressible fluid hydrodynamics [22]. Each phenomenon considered separately is accurately described in previous reports and can easily be studied. However, when combined, as during voiding, they constitute such an intricate set that to analyze individual recorded voiding and reconstruct the possible causes of dysfunction (e.g., compressive or constrictive urethral obstruction), elaborate software is needed, e.g., VBN® software. Upon gender and initial bladder volume being entered, the VBN® software allows voiding curves to be computed: flow rate and detrusor pressure vs. time. Two parameters describe the status of the urethra. The first, σ, characterized its effective cross-sectional area: a constrictive obstruction (a stricture) is characterized by σ < 1 and a gaping by σ > 1; the second parameter, γ, describes a local compressive obstruction exerted on the urethra (similar to the effect of an enlarged prostate in men). Parameter σ is without unit (multiplicative coefficient of the normal value); parameter γ represents a pressure in which the unit is centimeters of H2O. Detrusor force is characterized by a detrusor force parameter k (a normal detrusor is associated with k = 1). Any voiding depends on both urethral and detrusor parameters and on possible circumstantial parameters (fading detrusor excitation, delayed sphincter opening or incomplete relaxation…).
Appendix 2
To analyze the geometric obstructive effect of a 7-F urethral catheter, theoretical computations were performed, using the Valentini–Besson–Nelson (VBN) model [16] in well-defined conditions. Calculations were made first for a normal urethra (no compression or constriction) (Fig. 4, left) and second for a urethra with an external compression of 15 cm H2O (Fig. 4 right). In each situation, two values of detrusor contractility were tested: k = 1 (normal detrusor) and k = 0.3 (hypocontractile detrusor). The range of initial bladder volumes was 100–600 ml.
Theoretical computations of the effect of a 7-F urethral catheter on maximum flow rate vs. initial bladder volume. Left normal urethra, normal detrusor contractility (k = 1), and decreased detrusor contractility (k = 0.3). Right urethral compression of 15 cm H2O, normal detrusor contractility (k = 1), and decreased detrusor contractility (k = 0.3)
For Vini = 400 ml (near the mean value in our study), the maximum decrease of Qmax was 3.4 ml/s; it was observed for a normal urethra and a normal detrusor. In all other cases, the decrease was lower.
Rights and permissions
About this article
Cite this article
Valentini, F.A., Robain, G., Hennebelle, D.S. et al. Decreased maximum flow rate during intubated flow is not only due to urethral catheter in situ. Int Urogynecol J 24, 461–467 (2013). https://doi.org/10.1007/s00192-012-1856-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-012-1856-2