Abstract
Introduction and hypothesis
Two-year outcomes of treating cystocele with a polypropylene mesh (Perigee System® with IntePro®, AMS, Inc.) placed via a transobturator approach are reported.
Methods
A prospective, multicenter trial was conducted evaluating 114 women with ≥ stage II anterior wall prolapse defined using International Continence Society guidelines. Treatment success was defined as anterior stage ≤ I at a 24-month follow-up. Quality of life questionnaires were administered at baseline and follow-up. Complications were reported via adverse events.
Results
Efficacy at 24 months was 88.5% (77/87). Pelvic floor distress inventory, pelvic floor impact questionnaire-7, and pelvic organ prolapse/urinary incontinence sexual questionnaire were all significantly improved from baseline (p < 0.001). Complication rates reported were vaginal mesh extrusion 10.5% (12/114) and groin, pelvic, or vaginal pain 4.4% (5/114). Six subjects reported de novo dyspareunia. Out of the 49 subjects reporting dyspareunia at baseline, 15 were resolved postoperatively.
Conclusions
The Perigee System is an effective treatment to repair anterior wall prolapse with a low rate of complications through a 2-year follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelvic organ prolapse (POP) is a significant worldwide health issue for women. In the United States alone, an estimated 300,000 POP-related surgical procedures are performed annually [1]. The traditional anterior surgical repair of cystocele or anterior wall prolapse is associated with high failure rates [2] and may shorten or constrict the vaginal anatomy. Paravaginal repairs performed abdominally or laparoscopically may provide better anatomic support, however, do not appear to be more effective than traditional anterior repair [3].
The use of synthetic mesh is considered the gold standard for treatment of vault prolapse abdominally with sacral colpopexy [4]. More recently, grafts have been used vaginally to treat prolapse, including the anterior compartment, in attempts to try to reproduce the success that mesh has been shown to have with sacral colpopexy. Various methods have been described and most have been shown to have higher cure rates than traditional repairs [5]; however, they may require more complex dissections and surgical techniques compared to traditional repair. The management of using a mesh graft in the anterior compartment is also supported by a recent Cochrane review in 2008 that reported a higher rate of recurrent prolapse after anterior colporrhaphy than after mesh repair [6].
The transobturator space has been shown to be a safe space for the placement of tension-free tape slings for the treatment of stress urinary incontinence and has simplified the technique of this procedure [7, 8]. The space has also been more recently used to assist with anterior wall mesh placement. De Tayrac was the first to describe its use in cystocele repair by securing the anterior arms of a tension-free graft; however, no apical attachment of the graft was described [9].
The Perigee System (AMS, Inc.) was developed as a minimally invasive treatment option to place an anterior wall graft for cystocele repair with both distal and more apical attachments to the pelvic sidewalls. It contains four side-specific, helical needles designed for each anatomic pass through the obturator space to attach a graft with four arms to the pelvic sidewall distally at the level of the bladder neck and apically near the ischial spines. A retrospective pilot study has previously reported an 18-month cure rate of 93.5% using this procedure [10]. Gauruder retrospectively reported an efficacy rate of 93% [11], and Nguyen reported an anterior vaginal support rate of 87% at 1 year [12].
The primary objective of this study was to prospectively evaluate long-term outcomes in a multicenter clinical trial of the Perigee transobturator vaginal mesh kit system to treat ≥ stage II symptomatic anterior vaginal wall prolapse.
Materials and methods
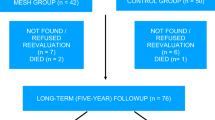
This is a prospective, multicenter, single-arm study. Subjects were recruited through normal clinical visits, and no advertising was conducted. Each study center received IRB approval, and each subject provided written informed consent prior to initiating any study-related activities. A total of 114 subjects were implanted and followed up at 6 weeks, three, six, 12, and 24 months. The study is still ongoing in a subset of the subjects up to 5 years.
Materials
The Perigee System with IntePro (AMS, Inc.), a polypropylene mesh kit was used in all study participants. The kit includes a monofilament macroporous polypropylene mesh comprised of four self-fixating adjustable arms with connectors for attaching to the needles. Each graft arm features a tensioning suture designed to maintain the integrity of the mesh arms as they are deployed through the anatomic passages and during the adjustment of the arms and the midline graft.
Methods
A total of 114 subjects underwent cystocele repair using the Perigee System. All subjects had symptomatic ≥ stage II anterior vaginal wall prolapse according to International Continence Society (ICS) guidelines (POP-Q) with planned repair and a total vaginal length > 4 cm but less than 11 cm. Concomitant reconstructive or incontinence procedures were allowed, except for hysterectomy. Other exclusion criteria included: current pregnancy, previous placement of an anterior wall graft, current pelvic infection, currently has a transobturator device in place or previous sacrospinous fixation, renal insufficiency, urinary tract obstruction, active or latent systemic infection, groin/vulvar/pelvic pain (unrelated to prolapse), undiagnosed vaginal bleeding, blood coagulation disorders, pelvic cancer/radiation to pelvic area, any genitourinary or bowel malignancy, enlarged inguinal lymph nodes, vaginal erosion or severe vaginal atrophy, vaginal fistula, urethral diverticulum or fistula, chronic inguinal or vulvar abscesses, hydradenitis suppurativa, signs of tissue necrosis, inability to be placed in lithotomy position, allergy to polypropylene, or predominant urge incontinence diagnosed by urodynamics. In this report, International Continence Society pelvic organ prolapse staging guidelines were followed, [13] with the exception of separating out the anterior measurements in order for staging to reflect the respective compartment under discussion.
The primary endpoint and treatment success were defined as ≤ stage I anterior vaginal wall prolapse (B a < −1.0 cm) at a 24-month follow-up. Failures who exited the study were carried forward. Secondary endpoints included the following validated quality of life (QOL) questionnaires: pelvic floor distress inventory (PFDI), pelvic floor impact questionnaire-7 (PFIQ-7) [14–16], and the pelvic organ prolapse/urinary incontinence sexual questionnaire (PISQ-12) [17]. Postoperative pelvic pain was measured using the Wong Baker visual analog pain scale [18]. Other observational data include procedure duration, estimated blood loss, and baseline vaginal maturation index [19]. Complications were reported via device-related adverse events. Sexual activity was determined through the responses to PISQ-12 and a survey question, “On average over the past 6 months, how often have you been sexually active?”
Surgical procedure
The polypropylene mesh was implanted in the anterior compartment to treat the cystocele using a vaginal and transobturator approach. Specifically, a small incision was made in the anterior wall, and the epithelium was dissected off of the bladder and out lateral to the pelvic sidewalls up to the level of the ischial spines. Hydrodissection was completed with a solution chosen by the surgeon. The mesh was attached to the pelvic sidewalls distally at the level of the bladder neck and apically, approximately 1.0 cm from the ischial spine, using needles that pass through groin incisions and the obturator space. The arms were adjusted in a tension-free manner through the groin incisions, and the apex or upper tail of the graft was cut by the surgeon to fit the length of the subject’s vagina. Upon final adjustment, the plastic sheaths covering the mesh arms were removed, excess mesh was cut at the incisions, and the vaginal incision was closed with a delayed absorbable suture. Concomitant reconstructive and incontinence surgical repairs were performed as needed during implant; however, the cystocele was neither reduced nor repaired with any sutures or other techniques under the graft. The type of anesthesia was per surgeon’s discretion.
Statistics
Summary statistics were calculated using conventional methods. Key factors for POP-Q staging and QOL scores were compared between baseline and follow-up with paired t test (if the difference was normally distributed) or Wilcoxon signed rank test (if the difference was not normally distributed). Primary endpoint was the percent of treatment success (anterior wall prolapse ≤ stage I) at a 24-month follow-up. Subjects who missed the 24-month follow-up with latest anterior wall prolapse stage II or higher were counted as treatment failures. Those who missed the 24-month follow-up with latest anterior wall prolapse stage I or lower were counted as missing. Exact 95% confidence interval of the percentage was calculated based on binomial distribution. Fisher exact test was used to explore whether or not the primary endpoint significantly differs between the subjects with and without concomitant vault suspension procedure. p value ≤ 0.05 was considered statistically significant. All statistical analyses were performed using SAS, version 9.1.3 (SAS Institute, Cary, NC, USA).
Results
Baseline subjects characteristic are noted in Table 1. The median follow-up was 23.5 months. Of the 114 subjects implanted, the 24-month follow-up data was obtained from 87 (76.3%) of the subjects. Of the 27 subjects missing 24-month follow-up visit, three failed previously and exited the study and two exited prior to any follow-up POP-Q. The remaining 22 subjects were lost to follow-up but had an anterior staging ≤ I at their last visit.
Concomitant procedures
Concomitant surgical procedures were performed in all but nine (7.9%) subjects with incontinence repair being the most common (Table 2).
Anatomical results
At baseline, anterior stages were stage II in 72 (63.2%) and stage III in 42 (36.8%) of implanted subjects. At 24 months, however, anterior stages were stage 0 in 58 subjects (66.7%); stage I in 19 subjects (21.8%); stage II in six subjects (6.9%); and stage III in one subject (1.1%). POP-Q measures: Aa and Ba were significantly improved from baseline. (Table 3)
Failure/success rates
Of the 87 subjects with a 24-month follow-up, 84 completed a POP-Q. Of the three missing POP-Q, two subjects refused their 24-month vaginal exam, and one subject was unable to come in for follow-up but completed the study questionnaires through the mail. A total of 77 of the 84 with POP-Q showed an anterior stage ≤ I. Three subjects with treatment failures who had exited the study prior to the 24-month visit were carried forward. Combining these three failures with the 84 subjects who had 24-month POP-Q tests resulted in a 24-month treatment success rate of 88.5% (77/87) with exact 95% CI (79.9 to 94.3).
Perioperative findings
Median surgical time for Perigee System was 26 min [min–max, 12–91] and estimated median blood loss as 50 mL [min–max, 4–400]. Two perioperative complications occurred: a bladder perforation during dissection was recognized and repaired with no sequelae, and a hematoma was evacuated intraoperatively, resulting in the subject requiring a transfusion of one unit of blood postoperatively.
Postoperative complications
Twelve subjects (10.5%) experienced vaginal mesh extrusion with median days-to-onset of 93 [min–max, 34–686]. No erosions were found in the surrounding viscera. Ten out of the 12 extrusions were resolved prior to 2 years follow-up. Eight of the 12 subjects with an extrusion required surgical correction which involved excision of the exposed mesh and secondary closure of the vaginal defect. One extrusion was resolved with vaginal estrogen cream application, and the other extrusion was resolved with trimming the exposed mesh fibers in office and the epithelium then healed over this area.
A total of 82% (94/114) of subjects were sexually active at baseline, compared to 75% (65/87) at 24 months. The overall dyspareunia rate was 49/94 (52%) pre-op versus 19/65 (29%) postoperatively. De novo dyspareunia was reported as a complication in six out of the 94 (6.4%) subjects who reported sexual activity at any time post-op. Of the six, two required surgical intervention and their dyspareunia resolved after this treatment. The first had a mesh extrusion that was treated with excision of the exposed mesh and closure of the epithelium and the second patient required removal of the graft secondary to tension on the lateral arms causing pain. One patient resolved with conservative treatment of vaginal estrogen cream alone, and three are ongoing. Of the three ongoing, none were noted as severe and none required any treatment to date. Of the 49 subjects with pre-op dyspareunia, 15 subjects had resolution of their dyspareunia postoperatively.
Four (3.5%) subjects reported de novo urge/incontinence; all received conservative treatment, three out of the four were resolved. Five (4.4%) subjects reported pain (two groin/pelvic, two vaginal, one with sitting); one out of the five incidents required additional surgery. This subject reported with new onset vaginal pain within the first month and had pain with palpation of the mesh arms vaginally. The decision was made by the surgeon to remove both the implant and a TOT sling that had been placed concomitantly as it could not be ascertained whether the pain was from the sling or the Perigee implant. Both implants were removed without complication and the subject’s symptoms resolved. At time of surgery, the Perigee arms were found to be under tension and pulling on the sidewalls.
Revisions
The overall revision rate for the Perigee mesh was 11.4% (13/114). See Table 4 for the details.
QOL questionnaire
Significant improvements were observed on the PFDI, PFIQ-7, and PISQ-12 QOL questionnaires across all applicable subscales (for each, p < 0.001; Table 5). The average Wong Baker score was 1.4 ± 1.9 at baseline, 0.9 ± 1.6 at 6 weeks, and 0.5 ± 1.5 at 6 months, p < 0.05 when comparing to the baseline.
Effect of concomitant vault suspension
Among 87 subjects whose primary endpoints were evaluated at 24-month, 58 underwent concomitant vault suspension and 29 didn’t. In the 58 subjects who had concomitant vault suspension, 40 had Apogee procedure; eight had laparoscopic sacral colpopexy; seven had ileococcygeus vault suspension; one had sacrospinous ligament suspension; and two indicated vault suspension but did not specify. Success rates in the groups with and without concomitant vault suspension were 91.4% and 82.8%, respectively, with p value of 0.291 (Table 6).
Discussion
This is an ongoing prospective observational cohort study in eight US centers, using a type I polypropylene mesh kit (Perigee) to treat stage II or greater cystocele using the transobturator approach.
At 24-month, the anatomic success rate was 88.5%. In addition, a significant improvement was seen in all QOL measurements (Table 5). These data are in agreement with previous work using the same device and technique for the treatment of anterior wall prolapse. Gauruder (2007) retrospectively reported an efficacy rate of 93% [11] and Nguyen (2008) reported an anterior vaginal support rate of 87% at 1 year [12].
Recently, concern over complications with vaginal mesh has been raised. Complications that may be related to the mesh itself, such as mesh vaginal extrusion, pain (vaginal, groin, buttock or leg), dyspareunia, infection, or fistula, have been reported in the literature [20, 21]. While these complications certainly are concerning, the overall rates of these complications are low in this study and in previously reported studies involving vaginal mesh and/or mesh kits. However, these complications should certainly not be overlooked or underplayed as the use of mesh can have complications that are unique to its use and can affect quality of life greatly. The current study was completed by surgeons with extensive experience in pelvic floor surgery and with the use of mesh in prolapse repair, and therefore, this may have had an impact on the low rate of complications encountered. It is difficult to answer whether our results can be translated to the general population of surgeons completing prolapse repairs, however, if the complications reported above seem to be happening more in the general population, then it may be secondary to surgeon experience or inexperience and possibly not the procedure itself. Prolapse repairs have advanced greatly over the years and may require a higher level of expertise and/or training to minimize risks and complications. Although traditional repairs may not pose the risk of the placement of a permanent implant, complications still do occur, including a very high failure rate which is a complication that cannot be ignored. Although a traditional anterior repair may be “easier” to perform with less risks of a permanent implant, are we really doing justice for our patients performing a procedure that we know has a high failure rate, is not anatomic, and not risk-free? Graft repairs seem to allow for an anatomic repair of the compartment with higher cure rates than traditional repairs and as long as complications can be kept to a minimum, such as with the current trial, the benefits seem to outweigh the risks. This certainly is an issue that we will have to continue to study and monitor.
Additionally, the type of mesh implanted and how it is implanted can impact complication rates. The use of a type I mesh, as in the current trial, seems to keep complications to a minimum. As in other series with this type of mesh, we did not have any infections that required mesh removal and it seems to be the best tolerated mesh used to date with very low rates of infection or rejection.
Vaginal mesh extrusion seems to be the most common complication seen with the use of synthetic mesh graft vaginally. This was also the case in the current study; however, the events were relatively easily handled by either trimming or excising a small portion of the mesh and closure of the epithelium. The incidence of mesh extrusions may be minimized by keeping the vaginal incision as small as possible, by making a slightly deeper dissection, and excising minimal vaginal epithelium. The rate of mesh extrusion in this study is consistent with other published reports of type I mesh complications at 3% to 12% [22, 23]. This type of complication may be reduced further in the future with newer techniques such as hydrodissection that allows a full-thickness dissection and the use of lighter, softer, less dense type I mesh.
Another concern for any vaginal repair of prolapse is dyspareunia. Recent studies have shown that vaginal mesh does not seem to have a negative impact on sexual function [24], and prospective comparative studies between mesh and traditional repairs in the anterior compartment have shown no significant difference in rates of dyspareunia [25]. Overall, data shows that vaginal mesh repair does not interfere with a healthy sex life [26]. Risk of dyspareunia or vaginal pain can be kept to a minimum by ensuring that the mesh lies flat in the space and is placed tension-free, i.e., the mesh arms penetrating the sidewalls should not be pulled too tight or create a “band” as this can cause pain with or without intercourse. The current study was consistent with previous studies [26] showing that the mesh does not seem to have a negative impact on sexual function. Although six subjects did report de novo dyspareunia, overall dyspareunia rates actually improved from 49/94 (52%) down to 19/65 (29%) postoperatively and 15 of 49 subjects that reported preoperative dyspareunia had a resolution of their symptoms. Additionally, PISQ scores significantly improved in subjects from pre- to postoperation, again indicating that the procedure did not seem to have an overall negative impact on sexual function. Of course, ideally, no patient would develop de novo dyspareunia; however, one has to remember that any repair for prolapse will have a baseline risk of dyspareunia and the goal is to attempt to keep this to a minimum. Additionally, in the current study, three out of six patients with dyspareunia had resolution of their symptoms, and although the other three had persistent symptoms, they did not need intervention. There have been other reports showing worsening sexual function following mesh use vaginally [27], however, this did not seem to be the case in the current trial.
The overall risk of postoperative new onset pain (vaginal, groin, buttock, or leg) was 4.4% in the current study. This too is consistent with what is seen with traditional repair without the use of mesh grafts (3.9% to 24.4%) [28]. All of these symptoms resolved with conservative treatment except one, which required surgical intervention.
The overall number of revisions was 13 (11.4%) in the trial. Among the 13, eight were for mesh extrusion (7.0%); two for recurrent cystocele (1.8%); one for dyspareunia (0.9%); one (0.9%) for bladder perforation; and one (0.9%) for urinary retention. These rates are consistent with the literature for vaginal prolapse repairs [29]. The majority of revisions were for mesh extrusions, which were easily handled and would be classified as minor complications. However, we do have to keep in mind that this is a complication, no matter how minor, that is unique to mesh repairs and one that we would not see in a traditional repair.
The study allowed vault suspension as a concomitant procedure, if indicated. A stratified analysis was performed to investigate whether concomitant vault suspension could assist anterior compartment repair, therefore, confounded the success rate. The results showed that the success rates with or without concomitant vault suspension were not statistically different. (Table 6) However, the study was not designed to answer the question. Therefore, it might be under-powered to detect differences in the outcome.
Patients with history of previous sacrospinous ligament suspension were excluded from the current study secondary to the possibility of encountering extensive scar tissue in the dissection, which could impact intraoperative complication rates. Patients with predominant urge incontinence diagnosed by urodynamics (i.e., severe detrusor instability) were excluded from the current study as to be able to better assess the impact of the procedure on causing or worsening urge symptoms.
Patient selection for the use of mesh in prolapse is also an issue that currently is under review in the field of pelvic surgery. Many may reserve mesh for patients with recurrent prolapse or larger prolapse such as stage III or IV. Others may argue for its use in all patients with symptomatic stage II or greater prolapse with the belief that if the native tissue has failed at all, it is not adequate for a long-term repair. We included all patients with symptomatic stage II or greater anterior wall prolapse in the study to be able to evaluate all parameters of this issue and gain knowledge in all subsets of patients with symptomatic prolapse.
The strengths of this study are that it is a prospective multicenter trial, with a long-term follow-up (currently 2 years). The study will be ongoing up to 5 years in a subset of subjects. Limitations of this study included its single-arm design; common use of concomitant procedures which may have affected the outcome of the results; and not allowing hysterectomy as a concomitant procedure and therefore, its conclusions should be drawn with appropriate caution. In addition, surgeons in the trial were experts with the particular procedure and/or anatomy; complications may have been kept to a minimum secondary to this variable, and therefore, the extrapolation of the results to general obstetrician, gynecologist, or urologists must be considered.
In conclusion, the 2-year follow-up data shows that the vaginal repair of anterior wall prolapse utilizing a type I mesh placed with needles passed through the transobturator space shows a high cure rate with minimal complications. The majority of subjects had improved quality of life scores, including sexual function. There were no major complications reported, such as mesh erosion into viscera or fistula formation, nor did any subject have an infection or abscess that required removal of the entire implant. Additional multicenter randomized control trials of type I mesh compared to traditional repairs are indicated to gain a more comprehensive understanding of complications, required treatments, and associated impact to quality of life for both types of procedures to provide more adequately council to patients about their options for reconstruction.
Abbreviations
- AMS:
-
American Medical Systems, Inc., Minnetonka, MN, USA
- AE:
-
Adverse event
- POP:
-
Pelvic organ prolapse
- POP-Q:
-
Pelvic organ prolapse quantification system
- QOL:
-
Quality of life
- PFDI:
-
Pelvic floor distress inventory
- PFIQ-7:
-
Pelvic floor impact questionnaire-7
- PISQ-12:
-
Pelvic organ prolapse/urinary incontinence sexual questionnaire
- IRB:
-
Institutional Review Board
- TOT:
-
Transobturator tape in the treatment of female stress urinary incontinence
- TVT:
-
Tension-free vaginal tape in the treatment of female stress urinary incontinence
- f/u:
-
Follow-up
References
Shah AD, Kohli N, Rajan SS, Hoyte L (2008) The age distribution, rate, and types of surgery for pelvic organ prolapse in the USA. Int Urogynecol J 19:421–428
Hiltunen R, Nieminen K, Takala T, Heiskanen E, Merikari M, Niemi K et al (2007) Low-weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol 110(2 Pt 2):455–462
Maher C, Baessler K (2006) Surgical management of anterior vaginal wall prolapse: an evidence based literature review. Int Urogynecol J Pelvic Floor Dysfunct 17:195–201
Nygaard IE, McCreery R, Brubaker L, Connolly AM, Cundiff G, Weber AM et al (2004) Abdominal sacralcolpopexy: a comprehensive review. Obstet Gynecol 104(4):805–823
Jia X, Glazener C, Mowatt G, MacLennan G, Bain C, Fraser C et al (2008) Efficacy and safety of using mesh or grafts in surgery for anterior and/or posterior vaginal wall prolapse: systematic review and meta-analysis. BJOG Oct 115(11):1350–1361
Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S (2007) Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev Jul 18;(3)
Mellier G, Benayed B, Bretones S, Pasquier JC (2004) Suburethral tape via the obturator route: is the TOT a simplification of the TVT? Int Urogynecol J Pelvic Floor Dysfunct 15:227–232
Davila GW, Johnson JD, Serels S (2006) Multicenter experience with the Monarc transobturator sling system to treat stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 17:460–465
de Tayrac R, Gervaise A, Chauveaud A, Fernandez H (2005) Tension-free polypropylene mesh for vaginal repair of anterior vaginal wall prolapse. J Reprod Med 50(2):75–80
Moore R, Miklos J (2009) Vaginal repair of cystocele with anterior wall mesh via transobturator route: efficacy and complications with up to 3 years follow-up. Advances in Urology 2009:743831, Epub 2009 Aug 24
Gauruder-Burmester A, Koutouzidou P, Rohne J, Gronewold M, Tunn R (2007) Follow-up after polypropylene mesh repair of anterior and posterior compartments in patients with recurrent prolapse. Int Urogynecol J Pelvic Floor Dysfunct 18:1059–1064
Nguyen JN, Burchette RJ (2008) Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol 111:891–898
Bump RC, Mattiasson A, Bo K et al (1996) The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 175:10–17
Barber MD, Kucibhatla MN, Pieper CF, Bump RC (2001) Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol 185(6):1388–1399
Barber MD, Walters MD, Bump RC (2005) Short forms of two condition specific quality of life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gyecol 193:103–113
Pessri Trial Group, Barber MD, Walters MD, Cundiff GW (2006) Responsiveness to the pelvic floor disorders inventory (PFDI) and pelvic floor impact questionnaire (PFIQ) in women undergoing vaginal surgery and pessary treatment for pelvic organ prolapse. Am J Obstet Gynecol 194:1492–1498
Rogers RG, Coates KW, Kammerer-Doak D, Khalsa S, Qualls C (2003) A short form of the pelvic organ prolapse/urinary incontinence sexual questionnaire (PISQ-12). Int Urogynecol J Pelvic Floor Dysfunct 14:164–168, discussion 168; Erratum in Int Urogynecol J Pelvic Floor Dysfunct. (2004) 15: 219
Wong D, Baker C (1988) Pain in children: comparison of assessment scales. Pediatr Nursing 14(1):9–17
Pauls RN, Mutema GK, Silva WA, Rooney C, Kleeman D, Karram MM (2006) Vaginal maturation index and female sexual function. Obstet Gynecol 107:102S–103S
Abdel-Fattah M, Ramsay I, West of Scotland Study Group (2008) Retrospective multicentre study of the new minimally invasive mesh repair devices for pelvic organ prolapse. BJOG 115:22–30
Marguilies R, Lewicky-Gaupp C, Fenner D, McGuire E, Clemens Q, Delancey J (2008) Complications requiring re-operation following vaginal mesh kit procedures for prolapse. Am J Obstet Gynecol December 678:e1–e4
Collinet P, Debodinance P, Ha Duc E, Lucot JP, Cosson M (2006) Transvaginal mesh technique for pelvic organ prolapse repair:mesh exposure management and risk factors. Int Urogyn J Pelvic Floor Dysfunct 17:315–320
Fatton B, Amblard J, Debodinance P, Cosson M, Jacquetin B (2007) Transvaginal repair of genital prolapse: preliminary results of new tension-free vaginal mesh (Prolift system)-a case series multicentric study. Int Urogyn J 18:743–752
Sentilhes L, Berthier A, Sergent F, Verspyck E, Descamps P, Marpeau L (2008) Sexual function in women before and after transvaginal mesh placement for pelvic organ prolapse. Int Urogyn J 19:763–772
Nieminen K, Hiltunen R, Heiskanen E, Takala T, Niemi K, Merikari M et al (2008) Symptom resolution and sexual function after anterior vaginal wall repair with or without polypropylene mesh. Int Urogyn J 19:1611–1616
Gauruder-Burmester A, Koutouzidou P, Tunn R (2009) Effect of vaginal polypropylene mesh implants on sexual function. European J Obstet Gynecol 142:76–80
Altman D, Elmer C, Kiilholma P, Kinne I, Tegerstedt G, Falconer C (2009) Sexual dysfunction after trocar-guided transvaginal mesh repair of pelvic organ prolapsed. Obstet Gynecol 113(1):127–133
Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR (2009) Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J 20(8):919–925
Diwadkar GB, Barber MD, MHS BF, Maher C, Eric Jelovsek J (2009) Complication and reoperation rates after apical vaginal prolapse surgical repair. Obstet Gynecol 113:367–373
Conflicts of interest
This study was sponsored by American Medical Systems Inc., Minnetonka, MN, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moore, R.D., Beyer, R.D., Jacoby, K. et al. Prospective multicenter trial assessing type I, polypropylene mesh placed via transobturator route for the treatment of anterior vaginal prolapse with 2-year follow-up. Int Urogynecol J 21, 545–552 (2010). https://doi.org/10.1007/s00192-009-1071-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-009-1071-y