Abstract
We compared safety and efficacy of Gynemesh PS® and Pelvicol® for recurrent cystocele repair. One hundred ninety patients were randomly divided into Gynemesh PS® and Pelvicol® groups and underwent tension-free cystocele repair. The Chi-square test was used to compare categorical variables, the paired t test for continuous parametric variables, and the Mann–Whitney test for continuous nonparametric variables. Ninety-six Gynemesh PS® patients and 94 Pelvicol® patients were studied. Mesh erosions occurred in 6.3% of Gynemesh PS® patients. No erosions were observed in Pelvicol® patients (p = 0.02). Objective cure was 71.9% for Gynemesh PS® and 56.4% for Pelvicol® (p = 0.06). Subjective cure was the same in both groups except for better sexuality in the Pelvicol® group. At 24 months follow-up, only Gynemesh PS® patients had mesh erosions. Anatomical outcome was similar in the two groups. Pelvicol® gave a better impact on voiding and sexuality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgery for pelvic floor disorders in women is widespread [1]. It has been estimated that about 11% of the female population will undergo surgery for prolapse during their lifetime, with approximately 30% of patients being reoperated within 4 years of the original surgery [2].
Many risk factors for pelvic organ prolapse (POP) recurrence have been suggested: inappropriate choice of procedure or suture materials, lack of surgical expertise, chronically increased intra-abdominal pressure, and, above all, low tissue quality [3]. Histological studies on patients with recurrent prolapse indicate that systemic collagen deficiency and increased amount of weaker type 3 collagen [4], combined with a decrease in type 1 collagen in the pubocervical fascia [5] and cervix [6], may also play a role. Increased collagen breakdown due to higher collagenase activity has also been noted [7]. Given the limitations of traditional surgery, the use of prosthetic materials has been suggested to reinforce or replace defective native tissue. Several types of meshes have been used for pelvic floor reconstruction and have become particularly popular in cystocele repair, which is where most recurrences occur. Among synthetic meshes, Mersilene® (Ethicon Inc., Somerville, NJ, USA) and especially Polypropylene (Prolene®, Ethicon Inc., Somerville, NJ, USA) have been widely used in recent years. Those are considered ideal synthetic meshes: Type-1, as classified by Amid [8], namely macroporous and monofilament meshes. These qualities have been associated with a reduction in the risk of bacterial harboring and graft infection, which may cause extrusion or erosion of the prosthetic material through the vaginal wall. Nevertheless, complications have been described also with type 1 synthetic materials. These include erosions, infection, pain syndromes, and dyspareunia [9].
In order to reduce the incidence of such complications, grafts of biologic origin have been developed as an alternative. Potential advantages of these materials include in vivo tissue modeling, histological similarity between graft and native tissue, and a reduced erosion rate. A disadvantage of biologic grafts could be low durability of tissue support, which may not last enough for optimal collagen ingrowth [10]. Based on previous knowledge, we hypothesized the occurrence of a possible lower complication rate for cystocele repair using Pelvicol® (CR Bard, Murray, NJ, USA), a porcine dermis graft, as compared to Gynemesh PS® (Ethicon Inc., Somerville, NJ, USA), a soft polypropylene mesh.
Several retrospective and nonrandomized studies have evaluated the use of different implant types in vaginal POP repair, but very few randomized trials have been published on the subject. In this prospective, randomized study on patients with recurrent cystocele, we evaluated the incidence of vaginal mesh erosion with Pelvicol® and Gynemesh PS® as a primary outcome. As secondary outcome measures, we evaluated the impact of surgery on quality of life and sexuality and we assessed anatomical results.
Materials and methods
Women with recurrent, symptomatic stage 2 or greater anterior vaginal wall prolapse (point Ba ≥ −1) planning to undergo secondary pelvic reconstructive surgery were enrolled in our study.
All patients underwent preoperative gynecological workup, which included:
-
History
-
Pelvic examination (the severity of POP was assessed in all patients by the Pelvic Organ Prolapse Quantification (POP-Q) staging system). [11]
-
Conventional urodynamic studies (uroflowmetry, filling cystometry, pressure–flow studies) with and without prolapse reduction with vaginal packing to diagnose occult stress urinary incontinence
-
Validated questionnaires including:
Methods, definitions, and units conform to the standards recommended by the International Continence Society [14].
Patients needing a concomitant anti-incontinence procedure and patients with diabetes mellitus or collagen disease were excluded from our study.
All patients underwent tension-free cystocele repair (TCR) [15] and levator myorrhaphy (LM). Concomitant hysterectomy was performed on 26 patients (27.6%) in the Pelvicol® group and 13 patients (13.5%) in the Gynemesh PS® group.
For TCR, patients were randomly assigned either to cystocele repair using Gynemesh PS® or to the same procedure using Pelvicol®. Randomization was done using a computer-generated list.
Gynemesh PS® is a knitted, monofilament, large-pore polypropylene, nonabsorbable mesh. It is made up of reduced diameter fibers knitted into a unique, patented design with 50% more flexibility than standard Prolene mesh. Gynemesh PS® was designed specifically for pelvic floor surgery and its advantage lies in its softness, suppleness, and lightness.
The Pelvicol® implant is derived from porcine dermis and has been used throughout the human body. Cellular skin components are removed, leaving the architecture of dermal collagen and elastin fibers intact. Mature collagen is stabilized by diisocyanate cross-linking, which makes the implant resistant to breakdown by naturally occurring collagenases. Pelvicol® has been demonstrated to be safe: noncytotoxic, nonallergenic, and nonmutagenic action of the implant has been noted.
Both Gynemesh PS® and Pelvicol® implants were trimmed and shaped in exactly the same way as required for the surgical technique adopted, which was identical in each case.
Surgical technique
TCR
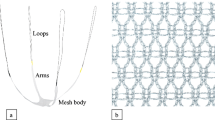
A midline incision is carried out on the anterior vaginal wall and the pubocervical fascia is dissected as for anterior colporrhaphy. The sheets of both the Pelvicol graft and the synthetic mesh are trimmed to an identical rounded shape, with two lateral wings. In each operation, the central, rounded part of the graft is positioned under the urinary bladder in a tension-free fashion, while its arms are inserted deep into the periurethral tissue on both sides towards the pubic bone (Fig. 1). A single fixating monocryl 2/0 suture is performed at the base of one wing of the mesh, at the periurethral level.
LM
Patients are placed in a dorsal lithotomy position. Midline posterior colpotomy extending from the vault to the perineum is performed. The prerectal fascia is widely dissected separating the vaginal wall from the underlying rectum. This dissection is extended to the ischiorectal fossa with the aid of Briesky retractors, until the sheath of the puborectalis muscle is visualized bilaterally at the superior edge of dissection. Using a single vicryl-2 suture, the right apex of the vaginal cuff is attached to the ipsilateral puborectalis sheath slightly further forward than the ischial spine (Fig. 2). The same suture then makes a loose stitch through the proximal end of the prerectal fascia. This procedure is then repeated on the left side using the same suture. The two ends of the suture are then tied, bringing together the upper parts of the two puborectalis sheaths.
Assuming a two-sided hypothesis test with a 5% type 1 error and 80% power, 90 patients in each group would be required to detect an absolute difference of 15% in reduction of complications. We sought to enroll 200 women in this clinical trial in order to allow for a 10% dropout rate.
All patients provided their informed consent to participate in our study. The study was approved by the Ethical Committee of our institution.
All patients were operated on by three different surgeons. The senior author (MC) was the primary surgeon in 140 of the cases and first assistant in 27 cases.
Postoperative workup was performed at 6 months, at 1 year, and then annually. Each visit included a pelvic examination and questionnaires. Conventional urodynamic studies were performed yearly.
We defined as anatomical cure point Ba < −1 (i.e., stage 0 or 1 according to the POP-Q system).
Descriptive statistics were mean ± standard deviation for parametric continuous variables (after confirmation of normal distribution with histograms, Q–Q plots and Skewness–Kurtosis test), median (minimum–maximum) for nonparametric continuous variables, and frequencies for categorical variables. The Chi-square test was used to compare categorical variables between preoperative and postoperative periods, the paired t test for continuous parametric variables, and the Mann–Whitney test for continuous nonparametric variables. We considered p < 0.05 to be statistically significant.
No financial assistance was received from any company in the design or execution of this study.
Results
From September 2003 until November 2005, a total of 200 patients with symptomatic recurrent anterior vaginal wall prolapse were enrolled. Of those, 190 patients were found to be eligible for our study: 96 were randomized to Gynemesh PS® and 94 were randomized to Pelvicol®.
No significant difference was found between groups in demographic data (Table 1), degree of POP, and clinical or urodynamic findings.
Sixty patients in the Gynemesh PS® group and 54 patients in the Pelvicol® group had previously undergone hysterectomy.
All patients underwent regional anesthesia and received antibiotics: ceftriaxone 2 g 1 h before surgery and 2 g/day for 5 days after surgery and metronidazole 500 mg twice a day for 5 days after surgery. Surgery lasted between 45 and 95 min (mean 68).
A Foley catheter was removed on the third postoperative day.
There were no intraoperative complications in either group. The hospital stay was 4–10 days in the Gynemesh PS® group (mean 4.5) and 4–12 days in the Pelvicol® group (mean 4.9), with p = 0.19. Resumption of spontaneous voiding occurred after 3–7 days (mean 3.7) in the Gynemesh PS® group and after 3–6 days (mean 3.4) in the Pelvicol® group (p = 0.34).
All patients completed the 2-year follow-up.
Anterior vaginal wall recurrence was observed in 27 patients in the Gynemesh PS® group (28.1%): in 26 patients, the recurrence was stage 2 while one patient had stage 3 recurrent prolapse. Forty-one patients in the Pelvicol® group (43.6%) showed recurrent cystocele, stage 2 in 39 patients, and stage 3 in the two remaining patients (Tables 2 and 3). The difference in anatomical outcome did not reach statistical significance (p = 0.06). None of the patients with recurrence was symptomatic enough to require reoperation for prolapse.
Nine women (six in the Gynemesh PS® group and three in the Pelvicol® group) had a stage 2 posterior vaginal wall recurrence. In six patients, a stage 2 recurrent prolapse at the upper vaginal segment was observed, all in the Pelvicol® group. In only two out of these six patients was a hysterectomy associated.
Mesh erosions through the vaginal mucosa were encountered in six patients in the Gynemesh PS® group (6.3%), while no cases of erosion were observed in the Pelvicol® group (p = 0.02). All cases of erosion were detected at the 6-month follow-up visit. The visible graft was excised, and the vagina was sutured with a continuous Vicryl 0 suture. No recurrence or subsequent related pelvic pain was observed. No other postoperative complications were detected in either group.
As regards symptoms, we observed in both groups a statistically significant reduction in voiding lower urinary tract symptom, in symptoms associated with pelvic organ prolapse, and in postmicturition symptoms (Table 4). There was also an improvement in overactive bladder symptoms; however, this was not statistically significant. Only two patients in the Gynemesh PS® group and one patient in the Pelvicol® group developed “de novo” stress incontinence after surgery.
A comparison between urodynamic parameters preoperatively and postoperatively at 2 years showed no statistically significant changes in the Gynemesh PS® group, while a significant decrease in detrusor pressure at maximum flow was observed in the Pelvicol® group following surgery (Table 5).
In the Gynemesh PS® group, analysis of quality-of-life questionnaires revealed significant improvement in the following domains: prolapse impact, social limitations, emotions, and severity measures. Patients in the Pelvicol® group reported a positive change in all domains, with the exclusion of physical limitations. Comparing the postoperative quality of life in both groups, we found a better impact of Pelvicol® in the following domains: social limitations (p = 0.04) and emotions (p = 0.02).
As regards sexuality, preoperative and postoperative PISQ-12 scores revealed no change in the Gynemesh PS® group (p = 0.31) and a statistically significant improvement in the Pelvicol® group (p = 0.03). Comparing postoperative data in the two groups, we observed a better impact of surgery on sexuality with Pelvicol® than with Gynemesh® (p = 0.03).
Discussion
The use of graft material in recurrent POP surgery has been adopted in clinical practice in order to decrease the postoperative recurrence rate which was seen with traditional techniques. Although surgery with graft augmentation has been suggested as a way of yielding superior anatomic outcomes [16, 17], this must be balanced against potential mesh-related complications such as erosion and dyspareunia. Following surgery with Marlex® mesh, the erosion rate has been reported at 1.4–25% [18, 19], whereas the rate of erosion associated with Prolene® mesh placement has been reported at 8–13% [20, 21]. There is some evidence that lower-weight polypropylene meshes may give an even lower erosion rate [22]. In this prospective, randomized study, comparing Gynemesh PS® and Pelvicol® grafts for the treatment of recurrent cystocele, vaginal mesh erosion was observed only in the Gynemesh PS® group. The occurrence of this complication in 6.3% of Gynemesh PS® patients is comparable to that reported by previous studies [16, 17]. The presence of concomitant vaginal hysterectomy in four out of six cases of erosion in our sample seems to confirm that hysterectomy might increase the risk of such complication, as reported by some authors [23]. Treatment of erosion included surgical excision of the visible graft and suturing of the vagina with continuous Vicryl 0. It should be noted that, beyond the need for surgical excision, erosion did not give rise to recurrence or subsequent pelvic pain.
We hypothesized that Pelvicol® biologic grafts would be less likely to cause erosion due to better tissue remodeling that would result from histological similarity between graft and native tissue at the surgical site [24]. The absence of erosions with Pelvicol® confirms our hypothesis and corroborates the results of some previous studies [25, 26]. Our results do not agree with those in a recent paper by Handel et al. [27] in which extrusion of porcine dermal graft through dehiscence of the anterior vaginal epithelium occurred in 22% of patients after surgical correction of cystocele. The authors postulated that perioperative bleeding accumulating deep to the graft could not drain from the vagina because of the nonporous nature of the graft, causing hematoma and eventual infection. We believe meticulous hemostasis and avoidance of extensive dissection of the anterior vaginal wall can reduce the rate of hematoma formation. Furthermore, “tension-free” positioning of the graft and avoidance of pubocervical fascia plication might better preserve blood perfusion to the vaginal skin, thus reducing the erosion rate [28].
The better impact of Pelvicol® on sexuality observed in our study might be explained by the fact that even low-weight polypropylene meshes may impair vaginal flexibility, which in turn causes discomfort during sexual intercourse [23]. The higher mesh flexibility at the level of the bladder neck given by Pelvicol® might also explain why we observed better results with Pelvicol® in urodynamics on the voiding phase compared with Gynemesh®.
This is the first prospective, randomized trial comparing the use of synthetic and biologic grafts in a selected population of patients with postoperative cystocele recurrence. Approximately 30% of patients have been reported to require repeated surgery for prolapse within 4 years of the original procedure. Nevertheless, review of the medical literature reveals paucity of data regarding surgical outcome of recurrent POP. In this regard, this study supplies important information which may affect clinical decisions, patient counseling, and informed consent.
With a follow-up of 24 months, we observed recurrent cystocele in 28.1% of Gynemesh PS® patients and in 43.6% of Pelvicol® patients. Difference between groups did not reach statistical significance (p = 0.06). The cross-linked structure of Pelvicol® may result in decreased durability of tissue support due to lower potential for collagen ingrowth, limited vascularization, and encapsulation of the graft [29]. This could explain the low success rate encountered in the Pelvicol® group. While judging the low anatomical success rates observed in both study groups, it should not be forgotten that the study population included only women with one or more previous surgery for POP, thus at highly increased risk for postoperative recurrence. It should also be noted that all patients in our sample underwent LM, a variation of the high levator myorrhaphy described by Lemack et al. [30] for suspension of the vaginal apex. The combination of a “tension-free” technique with a “tension-based” procedure such as LM may possibly play a role in the unsatisfactory anterior vaginal wall outcome.
Despite the somewhat disappointing anatomical outcome, analysis of P-QoL questionnaires resulted in good subjective outcome for patients in both study groups.
The impact of surgery on vaginal anatomy, on sexual and urinary function, and on the rate of mesh erosion might be influenced by the surgical approach, the associated procedures, as well as by the type of mesh. The technique for cystocele repair adopted in this study was TCR, described by our group in a previous report [15]. As a result, the outcome reported in the present study might not be predictive of the effect of the studied grafts when other surgical techniques, such as the popular transobturator approach, are adopted. This may limit the implications of our results for current clinical practice.
Recently, a biologic graft of porcine dermis, cross-linked but with a perforated structure, was developed and introduced in the market (Pelvisoft®, CR Bard, Cranston, RI, USA). The addition of perforations might allow better ingrowth of native tissue and incorporation of grafts, possibly overcoming the risk of encapsulation and yielding better tissue support.
Recently, surgical kits combining a polypropylene structure with biologic lining on the vaginal side of the graft have been developed and introduced in the market (e.g., Avaulta Plus® BioSynthetic Support System, CR Bard, Covington, GA, USA).
Those modifications, which combine a transobturator approach with biologic grafts, may become a safe and effective option for cystocele repair. The main disadvantage is the high cost of the biologic graft kits currently available.
Such innovations, aimed at delivering strong tissue support while yielding low erosion rates, should be the subject for future research and should be evaluated in the context of further prospective, randomized trials.
Abbreviations
- LM:
-
levator myorrhaphy
- POP:
-
pelvic organ prolapse
- POP-Q:
-
pelvic organ prolapse quantification
- PISQ-12:
-
Pelvic Organ Prolapse–Urinary Incontinence Sexual Questionnaire
- TCR:
-
tension-free cystocele repair
References
Boyles SH, Weber AM, Meyn L (2003) Procedures for pelvic organ prolapse in the United States, 1979–1997. Am J Obstet Gynecol 188:108–115
Olsen AL, Smith VJ, Bergstrom JO, Collinq JC, Clark AL (1997) Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 87:501–506
Birch C, Fynes MM (2002) The role of synthetic and biologic prostheses in reconstructive pelvic floor surgery. Curr Opin Obstet Gynecol 14:527–535
Norton P, Boyd C, Deak S (1992) Collagen synthesis in women with genital prolapse or stress urinary incontinence. Neurol Urodyn 11:300–301
Rechberger T, Postawsi K, Jakowicki JA (1998) Role of fascial collagen in stress urinary incontinence. Am J Obstet Gynecol 179(6Pt1):1511–1514
Wong MJ, Harmanli OH, Agar M (2003) Collagen content of nonsupportive tissue in pelvic organ prolapse and stress urinary incontinence. Am J Obstet Gynecol 189:1597–1599
Boreham MK, Miller RT, Schaffer JI, Word RA (2001) Smooth muscle myosin heavy chain and caldesmon exp in the anterior vaginal wall of women with and without organ prolapse. Am J Obstet Gynecol 185:944–952
Amid PK (1997) Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia 1:15–21
Ridgeway B, Chen CCG, Paraiso MFR (2008) The use of synthetic mesh in pelvic reconstructive surgery. Clin Obstet Gynecol 51:136–152
Le TH, Kon L, Bathia NN, Ostergard DR (2007) Update on the utilization of grafts in pelvic reconstruction surgeries. Curr Opin Obstet Gynecol 19:480–489
Bump RC, Mattiasson A, Bo K, Brubaker LP, DeLancey JO, Karskov P et al (1996) The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 175:10–17
Digesu GA, Santamato S, Kullar V, Santillo V, Digesu A, Cormio G et al (2003) Validation of an Italian version of prolapse quality of life questionnaire. Eur J Obstet Gynecol Reprod Biol 106:184–192
Roger RG, Coates KW, Kammerer-Doak D, Khalsa S, Qualls C (2003) A short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual questionnaire (PISQ-12). Int Urogynecol J 14:164–168
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U et al (2002) The standardisation of terminology of lower urinary tract function: report from the standardization sub-committee of the International Continence Society. Neurourol Urodyn 21:167–178
Cervigni M, Natale F, La Penna C, Panei M, Mako (2008) A transvaginal cystocele repair with polypropylene mesh using a tension-free technique. Int Urogynecol J Pelvic Floor Dysfunct 19:489–496
Hiltunene R, Nieminen K, Tarala T, Heiskanen E, Merikari M, Niemi K et al (2007) Low-weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol 110:455–462
Nguyen JN, Burchette RJ (2008) Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol 111:891–898
Julian TM (1996) The efficacy of Marlex mesh in the repair of severe, recurrent vaginal prolapse of the anterior midvaginal wall. Am J Obstet Gynecol 175:1472–1475
Flood CG, Drutz HP, Waja L (1998) Anterior colporrhaphy reinforced with Marlex mesh for the treatment of cystoceles. Int Urogynecol J Pelvic Floor Dysfunct 9:200–204
de Tayrac R, Gervaise A, Chauveaud A, Fernandez H (2005) Tension-free polypropylene mesh for vaginal repair of anterior vaginal wall prolapse. J Reprod Med 50:75–80
De Tayrac R, Deffieux X, Gervaise A, Chauveaud-Lambing A, Fernandez H (2006) Long-term anatomical and functional assessment of the trans-vaginal cystocele repair using a tension-free polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct 18:251–256
Debodinance P, Berrocal J, Clavè H, Cosson M, Garbin O, Jacquetin B et al (2004) Changing attitudes on the surgical treatment of urogenital prolapse: birth of the tension-free vaginal mesh. J Gynecol Obstet Biol Reprod 33:577–588
Deffieux X, de Tayrac R, Huel C, Bottero J, Gervaise A (2007) Vaginal mesh erosion after trans-vaginal repair of cystocele using Gynemesh or Gynemesh-Soft in 138 women: a comparative study. Int Urogynecol J 18:73–79
Jakus SM, Shapiro A, Hall CD (2008) Biologic and synthetic graft use in pelvic surgery: a review. Obstet Gynecol Survey 63:253–266
David-Montefiore E, Barranger E, Dubernard G, Detchev R, Nizard V, Darai E (2005) Treatment of genital prolapse by hammock using porcine skin collagen implant (Pelvicol). Urology 66:1314–1318
Meschia M, Pifarotti P, Bernasconi F, Magatti F, Riva D, Kocjancic E (2007) Porcine skin collagen implants to prevent anterior vaginal wall prolapse recurrence: a multicenter, randomized study. J Urol 177:192–195
Handel LN, Frenkl TL, Kim JH (2007) Results of cystocele repair: a comparison of traditional anterior colporrhaphy polypropylene mesh and porcine dermis. J Urol 178:153–156
Gauruder-Burmester A, Koutouzidou P, Rohne J, Gronewold M, Tunn R (2007) Follow-up after polypropylene mesh repair of anterior and posterior compartments in patients with recurrent prolapse. Int Urogynecol J 18:1059–1064
Davila GW, Drutz D, Deprest J (2006) Clinical implications of the biology of the grafts: conclusions of the 2005 IUGA Grafts Roundtable. Int Urogynecol J 17:S51–S55
Lemack GE, Zimmern PE, Blander DS (2000) The levator myorrhaphy repair for vaginal vault prolapse. Urology 56:50–54
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Natale, F., La Penna, C., Padoa, A. et al. A prospective, randomized, controlled study comparing Gynemesh®, a synthetic mesh, and Pelvicol®, a biologic graft, in the surgical treatment of recurrent cystocele. Int Urogynecol J 20, 75–81 (2009). https://doi.org/10.1007/s00192-008-0732-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-008-0732-6