Abstract
The goals of this study were to describe the surgical procedure of the transverse cystocele repair with uterine preservation using native tissue and to examine the surgical complications and short-term anatomical outcomes of this operation. Patients who underwent transverse cystocele repair with uterine preservation at our institution were identified by retrospective chart review for the interval from January 2001 to September 2006. Sixty-nine patients were identified. Median point for first postoperative visit was 6.1 weeks (range 3–101 weeks). Average age was 66.6 ± 13.1 years (range 33–89). Patients undergoing this procedure had no intraoperative complications and high frequency of initial anatomic success (defined as Baden–Walker halfway system grade 0 or 1 for anterior compartment) during a relatively short follow-up interval. Preoperatively, bladder grade averaged 2.6 with postoperative grade averaging 0.02. Based on our initial anatomical findings, we conclude that this surgical approach has merit for a subset of patients with adequate uterine support.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anterior vaginal wall prolapse can occur with or without uterine prolapse or vaginal cuff prolapse. If associated with uterine prolapse, the traditional surgical intervention involves hysterectomy; however, some patients experience anterior compartment prolapse without significant uterine prolapse. In those patients, hysterectomy can be avoided for women who desire uterine preservation as well as for those patients with increased risks of morbidity or decreased risk of recurrence due to advanced age or limited physical activity.
In the absence of premalignant or malignant uterine pathology, uterine preservation during reconstructive pelvic surgery is an option. This option, however, is supported by limited clinical data. Diwan et al. performed a Medline search of literature in English from 1966 to 2003 pertaining to uterine preservation in pelvic reconstructive surgery for uterovaginal prolapse [1]. Neither they nor we found descriptions of anterior colporrhaphy with only native tissue and uterine preservation. Their review focused on uterine support techniques including uterosacral suspensions, sacrospinous hysteropexies, and sacrohysteropexies; all have been noted by multiple authors [2–4]. These reports involve uterine preservation and support procedures for uterine prolapse. Behnia-Willison et al. described laparoscopic paravaginal repair with uterosacral hysteropexy or colpopexy with follow-up graft-reinforced anterior colporrhaphy for recurrent central defects [5].
Previously reported surgical treatments for anterior compartment prolapse are associated with significant persistence or recurrence rates despite concomitant procedures to address apical, transverse, and paravaginal support of the anterior segment [6–12]. Prior studies at our institution have reported using native tissues to provide good vaginal support; however, the anterior compartment has presented the greatest challenge for anatomic success [6–9]. When combined with hysterectomy or with post-hysterectomy vaginal cuff prolapse, our preferred technique for vaginal reconstruction utilizes the uterosacral ligaments to suspend the transverse portion of the connective tissues of the anterior and the posterior compartments, but we have also employed vaginal paravaginal repair, sacrospinous ligament suspension, and iliococcygeus suspension [6–10]. Morse et al. found in a retrospective review that, in patients undergoing prolapse surgery, vaginal paravaginal repair combined with anterior colporrhaphy did not improve anatomic or quality-of-life outcomes when compared with anterior colporrhaphy alone [11].
Some authors have reported modest improvements with the use of foreign materials. Others have concluded that the risks associated with those materials outweigh the modest (if any) improvement in recurrence rates of anterior prolapse. Weber et al. performed a randomized trial of three surgical techniques to address anterior vaginal wall prolapse [12]. They compared traditional or standard anterior colporrhaphy, standard anterior colporrhaphy with polyglactin 910 mesh, and ultralateral anterior colporrhaphy. The three groups provided similar cure rates of 30% to 46% [12]. Sand et al. found polyglactin 910 mesh to be useful in the prevention of recurrent cystoceles with their data demonstrating 43% recurrence without mesh and 25% with mesh augmentation [13].
Since 2001, the pelvic reconstructive surgeons at Scott and White Memorial Hospital and Clinic have performed cystocele repairs with uterine preservation in selected patients with adequate uterine support. No literature exists to describe this procedure or to report operative outcomes. In this study, we desired to describe the surgical procedure of the transverse cystocele repair with uterine preservation using native tissue and to examine the surgical complications and short-term anatomical findings of this operation.
Materials and methods
Patient population
Patients with symptomatic anterior vaginal wall prolapse and adequate uterine support were offered transverse cystocele repair with uterine preservation. The patient’s age, anticipated activity level, and comorbidities were considered. Younger patients with grade 1 uterine prolapse were less likely to have this procedure offered to them than were older patients. Preoperatively patients are examined in dorsal lithotomy position with a single-toothed tenaculum applied to the cervix. With the patient bearing down, gentle traction is applied to the tenaculum to assess uterine support; this is typically well tolerated by the patients. If the cervix prolapses to within 1 cm from the hymen, the patient is less likely to be offered this procedure. Patients with multiple comorbidities or advanced age are sometimes offered the procedure despite grade 2 cervical prolapse.
Post-void residual is evaluated with simple in-and-out catheterization. Urine dipstick indicates presence or absence of infection. Positive urine dipstick tests are followed with urine cultures. If a patient has urinary complaints including incontinence, subtracted cystometry is performed. In women with severe prolapse, continence is evaluated with the prolapse reduced. Patients demonstrating stress urinary incontinence are offered concurrent placement of mid-urethral slings.
Patients who are generally healthy are scheduled for outpatient procedures. Those with notable comorbidities or who are of advanced age may be monitored overnight for precautionary reasons. Patients are counseled in advance regarding the potential for catheter use and the voiding trials they will encounter prior to and potentially after hospital discharge.
Operative technique
Preoperative prophylactic intravenous antibiotics are administered to all patients. Using a tenaculum or Allis clamp on the cervix, an examination under anesthesia is performed prior to surgery to assess uterine/cervical support. Anterior and posterior compartments are evaluated as well. Anterior vaginal wall prolapse is present while apical support of the uterus remains adequate. Transverse separation of the pubocervical fascia is suspected when the anterior vaginal wall prolapses past the cervix and vaginal rugation is absent or diminished in the superior anterior vagina. After prepping and draping the patient, the bladder is drained.
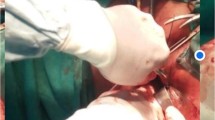
A series of Allis clamps is placed in the mid-longitudinal plane from the urethrovesical junction to the anterior lip of the cervix. A midline longitudinal incision is made in the vaginal epithelium. When a midurethral sling is placed, a separate suburethral incision is made. The vaginal epithelium is sharply dissected away from the underlying pubocervical connective tissue and bladder. The transverse portion of the pubocervical connective tissue is identified and grasped (Fig. 1a). It is attached to the cervical stroma 3 to 4 cm proximal to the external cervical os (Fig. 1b). Two 2-0 polyester suspensory sutures are passed through the lateral apical portions of the pubocervical connective tissue and the anterior cervical stroma. These two suspensory sutures are held for later approximation. Midline plication of the pubocervical connective tissue is performed with a series of simple side-to-side interrupted 2-0 polyester sutures to complete the anterior colporrhaphy (Fig. 1c). The two previously inserted suspensory sutures in the cervical stroma are then tied, thus supporting the transverse portion of the pubocervical connective tissue to the cervix (Fig. 1c). A third suspensory suture can be placed in triangular fashion in the midline of the plicated pubocervical connective tissue and the cervical stroma. If necessary, the vaginal epithelium is trimmed. It is closed in running locking fashion with 2-0 polyglactin 910 suture (Fig. 1d). The epithelium can be tacked to the pubocervical connective tissue and cervix with approximately every third throw in the vaginal epithelium closure at the surgeon’s discretion. Cystoscopy is performed to demonstrate ureteral efflux, to confirm bladder integrity, and to rule out the presence of a foreign body/suture in the bladder.
Anterior compartment dissection and reconstruction. a Apex of pubocervical connective tissue grasped with Allis clamps. b Pubocervical connective tissue retracted cephalad with anterior cervical stroma (black asterisk) exposed for suspensory suture placement. Two suspensory sutures are placed through the lateral aspect of the pubocervical connective tissue (white asterisk) then through the cervical stroma before being passed through the contralateral aspect of the pubocervical connective tissue. Suspensory sutures are held. Midline plication is performed. Suspensory sutures are then tied. A third suspensory suture can be placed in the midline after plication is performed. c Plicated pubocervical connective tissue with suspensory sutures previously placed through anterior cervical stroma. d Completed repair
Following the procedures, patients are sent to the recovery room with Foley catheter in place. Patients who are discharged on the day of surgery undergo voiding trials prior to discharge. Patients staying overnight undergo voiding trials on postoperative day 1. Prior to discharge, the bladder is backfilled with 300 ml of normal saline, and the catheter is removed. The patients are given up to 15 min to void. The voided volume is recorded. In-and-out catheterization is used to check post-void residual. Patients with post-void residual volumes of 150 ml or less are discharged home without a catheter. Those with post-void residual volumes exceeding 150 ml are offered clean intermittent self-catheterization. Some patients are unable to perform self-catheterization or simply prefer not to do so. Foley catheters are replaced for those patients. Any patient requiring catheterization is prescribed prophylactic oral antibiotics on a daily basis until return of adequate spontaneous voiding. Those who choose self-catheterization are taught proper hygiene as well as criteria for discontinuation—two consecutive post-void residuals of 150 ml or less. Patients discharged with Foley catheters typically return on postoperative day 1 or 2 for in-office voiding trial. If catheterization is still needed, the patient is encouraged to learn self-catheterization; those patients who choose to continue using in-dwelling catheters return for in-office voiding trials within 2 to 7 days.
Statistical analysis
The Scott and White Institutional Review Board provided study approval for this retrospective analysis. Patients who underwent transverse cystocele repair with uterine preservation by three physicians (BLS, TWM, PMY) were identified by retrospective chart review for the interval from January 2001 to September 2006. Data were extracted from the records of the preoperative assessments, surgical notes, anesthesia records, short-term (approximately 6-week) postoperative assessments, and any follow-up examinations available (Table 1). Data analysis was performed using Statistica (StatSoft, Tulsa, OK, USA) software. Analyses included summary of quantitative variables as means with standard deviations and ranges, summary of qualitative variables as percentages or median with quartiles, and percentage of patients with anatomical failure (defined as Baden–Walker halfway system grade 2, 3 or 4 [14]) in the anterior compartment of the vagina in relation to duration of follow-up using survival curve methods. Groups were compared using analysis of variance, Kruskal–Wallis test, and Pearson’s chi-square test.
Eighty-six percent (59 of 69) of patients were assessed for pelvic floor support preoperatively using the Baden–Walker halfway system [14]. Because only ten patients were evaluated using the pelvic organ prolapse quantification (POP-Q) system [15] compared to 59 using the Baden–Walker system, the ten POP-Q patient measurements were converted to the Baden–Walker system for statistical purposes.
Results
A total of 69 patients underwent transverse cystocele repair. Sixty eight had between 1 and 11 previous pregnancies (3.3 ± 1.9; mean ± SD) with an average of 2.9 ± 1.6 vaginal deliveries. The patients ranged in age from 33 to 89 (66.6 ± 13.1) years with body mass index (BMI) of 27.5 ± 5.0 kg/m2 (range 18 to 44 kg/m2). Fifty nine (86%) of these 69 were menopausal, but only 11 (16%) were using hormone replacement therapy. Twenty-six (38%) patients reported one or more previous pelvic surgical procedures, although only five patients had procedures related to pelvic support or incontinence including uterine suspension, tension-free vaginal tape, retropubic urethropexy, rectocele repair, and cystocele repair.
In addition to the transverse cystocele repair, other procedures were performed concurrently in 54 patients. These combinations of procedures were placed in five groups: A, limited to transverse cystocele repair; B, transverse cystocele repair with placement of tension-free vaginal tape; C, transverse cystocele repair and posterior repair with or without perineorrhaphy; D, transverse cystocele repair with placement of tension-free vaginal tape and posterior repair with or without perineorrhaphy; and E, transverse cystocele repair with other procedures that could include but were not limited to those referenced in groups A through D (Table 2).
Age and BMI did not vary for the various surgical groups, but the intraoperative parameters of duration (p < 0.0001) and estimated blood loss (p = 0.015) did differ (Table 2). Surgeries limited to transverse cystocele repair were of the shortest duration and involved the least amount of blood loss. Surgical grouping based on types of additional procedures was not related to the percentage of patients hospitalized (p = 0.11) or dismissed with urinary catheter in place (p = 0.34; Table 2). There were no intraoperative complications. An 81-year-old woman died suddenly secondary to cardiopulmonary arrest approximately 5 weeks following transverse cystocele repair and posterior colporrhaphy.
Additional analysis was performed to compare patients who underwent placement of tension-free vaginal tapes (TVT) to those who did not (Table 3). Patients who had TVTs placed were significantly younger (p = 0.003). No statistical difference was found for body mass index (p = 0.73), duration of surgery (0.16), estimated blood loss (p = 0.13), or patient hospitalization (p = 0.98). A trend toward significance was noted for urinary catheter removal prior to discharge. Seventy-nine percent of patients who underwent TVT placement were discharged without catheters compared to 94% of those who did not have TVTs placed (p = 0.06). Patients who did not have TVTs placed were found to have a higher preoperative grade of bladder prolapse compared to those who did undergo TVT placement (average Baden–Walker grade of 2.7 versus 2.1, p < 0.01).
Sixty of 69 patients (87%) passed their initial voiding trials and were dismissed without a catheter. Only three patients required catheterization for longer than 1 week. Nine patients were dismissed requiring urinary catheterization, and one additional patient returned on postoperative day 2 with incomplete bladder emptying. Of these ten patients, seven initially chose placement of in-dwelling catheter, while three chose to perform clean intermittent self-catheterization. Five of the ten had undergone placement of TVT along with transverse cystocele repair with or without posterior colporrhaphy. Three of the ten patients required catheterization for more than 1 week; two of those patients underwent TVT placement in addition to transverse cystocele repair. One of those two patients was still requiring intermittent self-catheterization at her 6-week postoperative visit; but, on her return visit at 3 months, she was no longer requiring intermittent self-catheterization. The three patients who required catheterization for longer than 1 week were all managed with clean intermittent self-catheterization. All eventually resumed spontaneous voiding function. Three patients were treated for postoperative urinary tract infections. Urinary symptoms before and after surgery were not charted using objective methods such as urodynamics or validated questionnaires thus preventing quantitative assessment of voiding parameters.
The Baden–Walker grades reported are listed in Table 4 for various intervals in relationship to the surgery. Forty-seven patients had Baden–Walker grades obtained for the anterior compartment at three time points (preoperatively, intraoperatively, and at the first postoperative visit). The Baden–Walker grades in the anterior compartment did not differ between preoperative and intraoperative assessments (mean of 2.6 versus 2.6, p = 0.66) but did differ between these values (mean of 2.6 versus 0.02, p < 0.0005) and the first follow-up visit in this subset of patients using analysis of variance with Duncan’s post hoc testing. Twelve patients had a grade of 2 for the cervix during the preoperative or intraoperative examinations or both. The average age of those patients was 71.8 years (range of 53–89). The median point for the first visit was 6.1 weeks with a range of 3 to 101 weeks following surgery in 55 patients evaluated using this scoring system.
Discussion
In the short term, suspending the plicated pubocervical connective tissue to cervical stroma provides an initially anatomically appropriate repair for anterior vaginal wall prolapse for patients with adequate cervical/uterine support. DeLancey previously demonstrated the anatomy and importance of the support structures of the anterior vagina including the pubocervical connective tissue [16]. The surgeons in this study recognize the importance of the transverse apex of the pubocervical connective tissue. Suspending the transverse portion of this tissue to the cervix along with midline plication has resulted in excellent anterior compartment support at the first postoperative visit (approximately 6 weeks). No lateral repairs were performed. We have found this procedure to be a safe and effective method to address anterior compartment prolapse in selected patients who may desire not to undergo hysterectomy or whose comorbidities might render them poor candidates to do so.
Candidates for transverse cystocele repair with uterine preservation are varied in their characteristics, but all must have adequate uterine/cervical support. In comparing preoperative and intraoperative cervical descent, we found no statistical difference. This is likely due to using a tenaculum to augment patient valsalva in assessing preoperative uterine/cervical prolapse. Also, by using the Baden–Walker system rather than the more precise POP-Q system, the prolapse grade may not vary despite a possible difference in prolapse of a few centimeters that may be noted during exam under anesthesia. Careful attention to preoperative and intraoperative assessment of each vaginal compartment must be undertaken to determine the appropriate repair. Our previously reported experience suggests that patients with good support at the 6-week postoperative visit will maintain good support in the future [9]. Additional follow-up, however, needs to be performed to assess the durability of this specific repair.
Minimal morbidity, blood loss, and operating time have been documented. Cystotomy and ureteral kinking are possible but have not occurred thus far; however, intraoperative cystoscopy is recommended. Operating time and estimated blood loss were increased in women who underwent combinations of procedures (groups B, C, D, and E), but postoperative dismissal and subsequent catheter use were not altered in these groups. Most patients were discharged on the day of surgery. Eleven patients were monitored for one or two nights for precautionary or pain-management reasons. Urinary continence status was evaluated only by simple inquiries at follow-up visits. No objective testing was performed, thus leaving only subjective patient responses as means of evaluation. Consequently, no objective conclusions can be reached regarding continence status. As noted in the results section, a trend toward significance was found regarding TVT placement and postoperative catheter requirements. Similar to the topic of continence, only simple inquiries were made at follow-up visits regarding pain and bowel function. In reviewing postoperative clinic information, bowel problems, urinary continence, and pain do not seem to have been problematic; however, data cannot be adequately presented to support this. With an average follow-up of only 6 weeks, dyspareunia cannot be evaluated in this study.
At our institution, this procedure is more likely to be performed for older women than for younger women. Many older women have comorbidities that encourage the implementation of less invasive techniques when surgical options are employed. Also, the durability of this procedure is not yet known. Younger patients are more likely to place increased stress on pelvic floor repairs through greater levels of physical activity and longevity. While the durability of repairs is important for every patient, it is a greater concern in younger women. Of the 12 women with a preoperative or intraoperative cervical grade of 2 who underwent the procedure, 9 were age 68 or older with an average age of 71.8. Advanced age and comorbidities were strong factors in choosing this procedure. Of these 12 patients, the cervical grade of 2 was typically noted with traction placed on the cervix which usually brought the cervix to within 1 cm proximal to the hymen.
There are limitations of this study. It is a retrospective chart review with small sample size and short-term follow-up of anatomic outcomes only. Furthermore, validated questionnaires were not used to evaluate patient symptoms. Some surgeons choose to use graft materials in pelvic reconstructive surgery. Reports of limited success and continued complications with grafts persist [12, 13, 17]. Baessler and Maher reviewed mesh-related complications and found erosion and dyspareunia rates up to 26% and 38%, respectively [18]. They noted that data on synthetic meshes in vaginal reconstruction were inadequate and that mesh should be used only in clinical trials. Other authors have found mesh to be safe and effective both with and without uterine preservation, although some have noted that hysterectomy increases the erosion rate [19–22]. Consequently, newer techniques for mesh implantation with uterine preservation are being investigated in order to decrease mesh complications. Despite the popularity for synthetic or biologic materials, sparse clinical evidence is available regarding procedural safety, efficacy, and complications [23].
In conclusion, the early results of this procedure are promising and provide another alternative for anterior compartment management when adequate uterine/cervical support exists. Given that this is not a technically difficult procedure, one of the primary variables rests with patient evaluation. The anterior, apical, and posterior vaginal compartments must be accurately assessed in order to determine the proper procedure for any patient with pelvic organ prolapse. Additionally, the patient’s comorbidities, expectations, and visceral and sexual functions must be taken into consideration.
References
Diwan A, Rardin CR, Kohli N (2004) Uterine preservation during surgery for uterovaginal prolapse: a review. Int Urogynecol J 15:286–292
Krause HG, Goh JT, Sloane K, Higgs P, Carey MP (2006) Laparoscopic sacral suture hysteropexy for uterine prolapse. Int Urogynecol J 17(4):378–381
Barranger E, Fritel X, Pigne A (2003) Abdominal sacrohysteropexy in young women with uterovaginal prolapse: long-term follow-up. Am J Obstet Gynecol 189(5):1245–1250
Dietz V, de Jong J, Huisman M, Schraffordt Koops S, Heintz P, van der Vaart H (2007) The effectiveness of the sacrospinous hysteropexy for the primary treatment of uterovaginal prolapse. Int Urogynecol J 18(11):1271–1276
Behnia-Willison F, Seman EI, Cook JR, O’Shea RT, Keirse MJ (2007) Laparoscopic paravaginal repair of anterior compartment prolapse. J Minim Invasive Gynecol 14(4):475–480
Shull BL, Bachofen C, Coates KW, Kuehl TJ (2000) A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol 183(6):1365–1374
Shull BL (1999) Pelvic organ prolapse: anterior, superior, and posterior vaginal segment defects. Am J Obstet Gynecol 181(1):6–11
Shull BL, Benn SJ, Kuehl TJ (1994) Surgical management of prolapse of the anterior vaginal segment: an analysis of support defects, operative morbidity, and anatomic outcome. Am J Obstet Gynecol 171(6):1429–1439
Shull BL, Capen CV, Riggs MW, Kuehl TJ (1992) Preoperative and postoperative analysis of site-specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol 166:1764–1768
Shull BL, Capen CV, Riggs MW, Kuehl TJ (1993) Bilateral attachment of the vaginal cuff to iliococcygeus fascia: an effective method of cuff suspension. Am J Obstet Gynecol 168(6):1669–1677
Morse AN, O’dell KK, Howard AE, Baker SP, Aronson MP, Young SB (2007) Midline anterior repair alone vs anterior repair plus vaginal paravaginal repair: a comparison of anatomic and quality of life outcomes. Int Urogynecol J Pelvic Floor Dysfunct 18(3):245–249
Weber AM, Walters MD, Piedmonte MR, Ballard LA (2001) Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol 185(6):1299–1306
Sand PK, Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, Goldberg R (2001) Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Am J Obstet Gynecol 184(7):1357–1364
Baden W, Walker T (1992) Surgical repair of vaginal defects. In: Fundamentals, symptoms and classification. Philadelphia, Lippincott-Raven, pp 9–23
Bump RC, Mattiasson A, Bo K, Brubaker LP, DeLancey JO, Klarskov P, Shull BL, Smith AR (1996) The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 175(1):10–17
DeLancey JOL (2002) Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol 187:93–98
Vakili B, Huynh T, Loesch H, Franco N, Chesson RR (2005) Outcomes of vaginal reconstructive surgery with and without graft material. Am J Obstet Gynecol 193(6):2126–2132
Baessler K, Maher CF (2006) Mesh augmentation during pelvic-floor reconstructive surgery: risks and benefits. Curr Opin Obstet Gynecol 18(5):560–566
Bai SW, Kim EH, Shin JS, Kim SK, Park KH, Lee DH (2005) A comparison of different pelvic reconstruction surgeries using mesh for pelvic organ prolapse patients. Yonsei Med J 46(1):112–118
Costantini E, Mearini L, Bini V, Zucchi A, Mearini E, Porena M (2005) Uterus preservation in surgical correction of urogenital prolapse. Eur Urol 48(4):642–649
Nicita G, Li Marzi V, Filocamo MT, Dattolo E, Marzocco M, Paoletti MC, Villari D (2005) Uterus-sparing vaginal surgery of genitourinary prolapse employing biocompatible material. Urol Int 75(4):314–318
Hiltunen R, Nieminen K, Takala T, Heiskanen E, Merikari M, Niemi K, Heinonen PK (2007) Low-weight polypropylene mesh for anterior vaginal wall prolapse. Obstet Gynecol 110(2 Pt 2):455–462
Gangam N, Kanee A (2007) Retroperitoneal hemorrhage after a vaginal mesh prolapse procedure. Obstet Gynecol 110(2 Pt 2):463–464
Acknowledgements
The authors thank Monika Hanusch for technical assistance. This work was supported by the Baden Family Center, the Noble Centennial Endowed Chair (TJK), and the Scott, Sherwood and Brindley Foundation.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huffaker, R.K., Kuehl, T.J., Muir, T.W. et al. Transverse cystocele repair with uterine preservation using native tissue. Int Urogynecol J 19, 1275–1281 (2008). https://doi.org/10.1007/s00192-008-0629-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-008-0629-4