Abstract
This study was performed to evaluate the efficacy and complications of the posterior intravaginal slingplasty (IVS). A retrospective chart review was performed. Ninety IVS procedures were performed from January 2004 to June 2005. The group consisted of predominantly nonsmoking, Caucasian, postmenopausal women with a median age of 62 years and a median parity of 3. The mean follow-up was 33 ± 23.2 weeks with a median of 31.9 weeks. There were no intraoperative bladder, bowel, or vascular injuries. Overall, 11 out of 90 patients developed recurrent or de novo prolapse; 4.4% of these had recurrent apical prolapse. There was a 17.8% incidence of mesh erosion. Only 1 of the 11 patients with recurrent prolapse had concomitant mesh erosion. The procedure demonstrated an unacceptably high erosion rate. The adoption of newer mesh techniques based on the slingplasty concept or the use of the multifilament polypropylene tape should be scrutinized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The lifetime risk of surgery for pelvic organ prolapse for women in the United States is estimated to be 11% [1]. The success of surgical repair for prolapse depends in part on the location of the defect leading to it. The vaginal apex is supported by the cardinal–uterosacral ligament complex as described by De Lancey [2]. The mechanism of apical prolapse is multifactorial, including muscles and connective tissue. The detachment of the cardinal–uterosacral ligament complex contributes to the descensus of the uterus or the vaginal vault after a hysterectomy. There is no consensus as to the optimal route for correction of vaginal vault prolapse.
The two common approaches to correct vaginal vault prolapse are the abdominal sacral colpopexy (ASC) and the sacrospinous ligament fixation (SSLF). In a comparison of these two procedures, the ASC was associated with a longer operating time, a slower return to activities of daily living, and a greater cost than the SSLF [3]. The SSLF demonstrated a higher rate of failure, and resulted in de novo cystocele formation, which was assumed to have resulted from a change in vaginal axis [4–7]. The vaginal approach may be a desirable alternative for elderly patients with multiple comorbidities or younger patients who desire a faster return to daily activities.
The posterior intravaginal slingplasty (IVS) technique was introduced to provide an anatomic repair of vaginal vault prolapse using permanent mesh with the benefits of a transvaginal approach. The posterior IVS (IVS Tunneler, Tyco U.S. Surgical, Norwalk, CT, USA) was first described by Petros as a minimally invasive approach to repair the vaginal vault. The posterior IVS uses a multifilament polypropylene tape to act as a neoligament. The tape replaces or reinforces the cardinal–uterosacral ligament complex to suspend the vaginal vault in a tension-free manner [8]. There are few studies that report the success rate and complications of this procedure.
The purpose of this study was to evaluate the short-term efficacy and complication rates associated with the posterior IVS procedure as performed at our institution.
Materials and methods
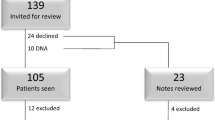
Approval for this study was obtained through the St. Louis University Institutional Review Board. The charts of women who underwent the posterior IVS procedure from January 2004 to June 2005 at St. Louis University were reviewed. All patients had a directed history, physical examination, postvoid residual measurement, and preoperative urodynamics with the prolapse reduced. Cystoscopy was performed on those patients with mixed incontinence and for selected indications such as hematuria, recurrent infection, and previous surgery. The patients underwent the posterior IVS procedure by one of three attending urogynecologists with the assistance of a resident or urogynecology fellow. The patient was offered a posterior IVS procedure if there was apical prolapse that descended to the midvagina or beyond on pelvic examination. The procedure was offered either as the primary procedure or in conjunction with another planned vaginal procedure of the other compartments. The patient's hospital and clinic charts were reviewed for demographics, prior surgical history, grade of prolapse [9], indications for surgery, duration of postoperative follow-up, perioperative complications, concomitant surgical procedures, estimated blood loss, and hospital length of stay.
The primary outcome variable was success of the surgery defined as the apex suspended at or above the midpoint of the vagina. The primary outcome was defined as this level to be consistent with the initial indications for the surgery, i.e., patients were offered the posterior IVS when the apex descended to the midvagina or beyond on the pelvic examination. The secondary outcomes included: failures in other vaginal compartments (de novo prolapse), perioperative complications, and surgical site infection. Other compartment failures were defined as any vaginal wall compartment (other than the apex) descending further than 1 cm from the hymenal ring. Mesh erosion was classified as visible extrusion of the IVS tape at any site; practically, this only occurred through vaginal epithelium.
Surgical technique
All three attending urogynecologists performed the procedure in a similar fashion. The posterior IVS was performed by making a vaginal midline incision with a scalpel in the posterior or anterior compartment depending upon the defect location. The vaginal incision was extended toward the apex with care to leave at least a 2-cm strip of vaginal epithelium intact. This was done to provide an intact surface for mesh attachment and limiting its exposure to the vaginal incision. The vagina was hydrodissected using a local anesthetic with epinephrine. Hemostasis was obtained with electrosurgical cautery or interrupted sutures. A small stab incision was made in each buttock 3 cm lateral and inferior to the anus. The IVS tunneler trocar was inserted through the buttock incision. It was directed through the ischiorectal fossa to pierce the iliococcygeus muscle 1 cm caudal to the ischial spine and emerge through the vaginal incision at the vaginal apex. The trocar was guided by the surgeon's finger placed in the vagina or rectum. The IVS tape was attached to the trocar and brought out through the buttock incision. The procedure was performed on each side in a similar fashion. The tape was attached at the vaginal apex with a polyglactin suture (Vicryl, Johnson & Johnson Gateway, Irvine, CA, USA). Concomitant procedures for incontinence and prolapse were performed before adjusting the tension on the IVS tape. The excess tape was excised at the buttock below the skin, and the vaginal epithelium was closed using a 2-0 or 3-0 polyglactin suture (Vicryl, Johnson & Johnson Gateway, Irvine, CA, USA). A rectal exam was performed at the end of the procedure to confirm the integrity of the rectum. The vagina was packed with gauze that was removed on postoperative day 1. Graft material was used to support additional vaginal compartments based on the surgeon's preference. The various graft materials used included polyglactin mesh (Vicryl, Johnson & Johnson Gateway, Irvine, CA, USA), bovine pericardium (Veritas Collagen Matrix, Synovis Surgical, St. Paul, MN, USA), and porcine dermal acellular collagen matrix (PelviSoft BioMesh, CR Bard, Cranston, RI, USA). All patients received a single intravenous dose of a prophylactic antibiotic with cefazolin, cefotetan, or clindamycin. All patients received oral stool softeners, were given strict lifting precautions, and were advised to maintain pelvic rest for 6 weeks.
Statistics
Descriptive statistics were used to analyze data. The Pearson chi-square test was used to analyze categorical variables. A Kaplan–Meier life table analysis was performed to analyze the time to mesh erosion. All analyses were performed with the SPSS 13.0 software (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
Results
Ninety patients underwent an IVS procedure over an 18-month period. The women ranged in age from 32 to 86 years with a median of 62 ± 12 years. The majority of the patients were Caucasian (89%), nonsmokers (89%), postmenopausal (89%), had an average body mass index of 28.2 ± 5.3 kg/m2, a median parity of 3 (0–10), and 48% were reported to be sexually active. Eighty percent of the group had a prior hysterectomy and 29% (n = 26) had also undergone prior prolapse surgery. Four (15%) of these 26 patients had a prior vaginal vault repair.
The mean follow-up was 33.0 ± 23.2 weeks with the median being 31.9 weeks. The majority of patients had grade III prolapse (73%). The women in the group had a concomitant diagnosis of urodynamic stress incontinence in 45.5% of which 7.8% had occult stress urinary incontinence, urge urinary incontinence in 5.5%, mixed urinary incontinence in 14.4%, and fecal incontinence in 5.6%. Postoperatively, no patients developed occult stress urinary incontinence and one patient developed persistent stress urinary incontinence. Of the patients who had preoperative mixed incontinence, 7/13 (54%) had resolution of the urge incontinence while only 1/5 (2%) who had urge incontinence alone had resolution of symptoms. All patients underwent concomitant prolapse procedures and 60% had an antiincontinence procedure. The details of the concomitant procedures, estimated blood loss, and hospital stay are noted in Table 1. There were no intraoperative bladder, bowel, or vascular injuries and no intraoperative blood transfusions were administered.
Four patients had recurrent prolapse of the apical compartment with an overall short-term success rate of the posterior IVS at 95.6%. There were six recurrent anterior wall prolapses and one new development of posterior wall prolapse. Each of the recurrent prolapse occurred in separate patients.
There was an 18% incidence of mesh erosion. The mean time to mesh erosion was 30.2 ± 22.7 weeks with a median of 28 weeks; however, mesh erosion did occur late in the postoperative period, as illustrated in Fig. 1. One case of mesh erosion was identified 2 years after surgery. A multivariable analysis found no association between mesh erosion and the use of graft augmentation, a concomitant hysterectomy procedure, or the attending surgeon performing the procedure. The incidence of mesh erosion was examined in 6 months intervals from the first performed posterior IVS procedure at St. Louis University. There was no significant difference in erosion rates over these time periods. In examining the association between surgical failure and mesh erosion, we found only 1 of the 11 patients with recurrent prolapse who also experienced mesh erosion.
Of the 16 patients with mesh erosion, 14 had the mesh initially excised in the office, 1 spontaneously healed, and 1 patient did not return after the diagnosis was made. The majority of the mesh erosions were treated with one office visit (10/16). Four patients were returned to the operating room for mesh excision after a failed excision in the office. There was one patient who required three office visits and two operative procedures for a nonhealing sinus tract at the site of mesh erosion.
The other postoperative complications are listed on Table 2. One patient developed postoperative retroperitoneal hematoma and had a blood transfusion. The caused of the bleeding was later discovered to be undiagnosed idiopathic thrombocytopenia purpura. One of the three abscess cases listed was a vaginal cuff abscess. It resolved after outpatient surgical incision and drainage. The other two involved the IVS tape; there were no sequelae once the tape was removed and the abscess drained. The one case of buttock cellulitis was associated with one of the buttock abscesses, which required intravenous antibiotics after incision and drainage. One patient had an anal fissure, but this resolved with stool softeners. The patients with apical scarring also had dyspareunia, and vaginal dilators were used and eventually underwent outpatient surgical revision. One of the four patients with apical scarring had a transobturator sling removed (ObTape, Coloplast, Minneapolis, MN, USA), which led to the resolution of her dyspareunia. Three of the patients with buttocks pain had spontaneous resolution at their 6-week postoperative visit. One had chiropractic manipulation and later resolved.
Discussion
Like many new procedures, there are few peer-reviewed studies assessing the posterior IVS technique. In the five studies published using the similar posterior IVS tape and technique, the success rates ranged from 91% to 98% with follow-up of 12 to 18.7 months. The incidence of mesh erosion ranged from 0% to 10%. The earlier studies had fewer numbers of patients than our current study (42–44) [10–12]. A recent study by Neuman and Lavy reported on the follow-up of 140 patients and showed a 10% incidence of mesh erosion [12].
This study reports our experience with the posterior IVS technique. The success rate was comparable to previous reports; however, our patients experienced a higher incidence of mesh erosion. Erosion of the mesh and mesh excision did not appear to adversely affect the surgical success rates.
An examination of the factors that might affect the risk of mesh erosion found no significant association with the performance of a concomitant hysterectomy, the use of graft augmentation, or the point during the procedure's “learning curve” when the surgery was performed. An examination of our surgical technique found no significant deviation from the original description of Petros et al. There appeared to be no pattern to the location of the posterior IVS mesh erosion. We hypothesized that performing concomitant procedures in different compartments could lead to the devascularization of the tissue around the apex and then to dysfunctional healing around the posterior IVS mesh. This theory is less plausible when it is considered that the other compartments healed well and we did not find rejection of other graft materials. This leads us to postulate that the inherent nature of the tape and/or the path that it travels contributes to the high erosion rate.
The stiff and inelastic nature of synthetic slings such as the IVS sling has been studied. It is speculated that these materials may not incorporate well into its host tissue [13]. A rabbit model comparing the IVS tape to four other graft materials concluded that the IVS tape had a similar tissue reaction at the beginning of transplantation, but had the weakest tissue attachment capacity [14]. A 17% incidence of mesh erosion has been reported using multifilament polypropylene tape in the midurethra (anterior IVS) for stress urinary incontinence [15].
The path of the sling may contribute to the increased mesh erosion. The tape is inserted near the anus, an area, which is considered a contaminated surgical site, and passes through the ischiorectal fossa. This may cause a low-grade inflammatory response in the ischiorectal fossa leading to a foreign body response and eventually to tape extrusion. Cultures were not obtained on the excised tape and we cannot comment on whether a low-grade infection may have contributed to the tape extrusion, although no patient demonstrated systemic signs of infection.
The posterior IVS is a minimally invasive novel approach to correcting apical prolapse by way of the vaginal route. Besides restoring the normal apical anatomy, it also has shown effects for curing symptoms of urgency and nocturia [10]. As the focus of our study was on the anatomical success for apical defect repair, we did not specifically assess these sensory symptoms. As there is a high association of urinary incontinence with prolapse, we did assess incontinence postoperatively. Patients with preoperative mixed urinary incontinence were more likely to have resolution of the urge component compared to those with only urge urinary incontinence. The reason for this difference is uncertain.
While the posterior IVS offers a more anatomical repair than the SSLF [16] and with potentially fewer complications and faster return to daily activities than the ASC, our experience with the high mesh erosion rate makes it less likely that we will continue to offer patients the posterior IVS. Despite the fact that most tape erosions were excised in the office, patient satisfaction with persistent discharge, bleeding, and multiple office visits makes it less appealing. In addition, the removal of the tape raises a real concern about the risk of recurrent prolapse as the longest follow-up data on the posterior IVS is 2 years [10]. There is a recent study, which suggested that the depth of placement of the anterior IVS mesh may have an impact on the erosion rate [17]. This surgical technique could potentially be explored in future posterior IVS study.
This study shows that the short-term success rate of the posterior IVS procedure is similar to other vaginal apical prolapse correction procedures. The procedure, however, demonstrated an unacceptably high tape erosion rate. The adoption of newer mesh techniques based on the slingplasty concept or the use of the multifilament polypropylene tape should be scrutinized. A randomized controlled trial comparing posterior IVS to other apical prolapse corrections is warranted.
References
Olsen AL, Smith VJ, Bergstrom JO, Collin JC, Clark AL (1997) Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 89:501–506
DeLancey JO (1992) Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol 166:1717–1724
Maher CF et al (2004) Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol 190:20–26
Benson J et al (1996) Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol 175:1418–1422
Paraiso MF et al (1996) Pelvic support defects and visceral and sexual function in women Treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol 175:1423–1431
Shull BL et al (1992) Preoperative and postoperative analysis of site-specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol 166:1764–1771
Holley RL et al (1995) Recurrent pelvic support defects after sacrospinous ligament fixation for vaginal vault prolapse. J Am Coll Surg 180:444–448
Petros PE (2001) Vault Prolapse II: restoration of dynamic vaginal supports by infracoccygeal sacropexy, an axial day-case vaginal procedure. Int Urogynecol J 12:296–303
Baden WF, Walker TA (1972) Genesis of the vaginal profile: a correlated classification of vaginal relaxation. Clin Obstet Gynecol 15:1048–1054
Farnsworth BN (2002) Posterior intravaginal slingplasty (infracoccygeal sacropexy) for severe posthysterectomy vaginal vault prolapse—a preliminary report on efficacy and safety. Int Urogynecol J 13:4–8
Jordaan DJ, Prollius A, Cronje HS, Nel M (2006) Posterior intravaginal slingplasty for vaginal prolapse. Int Urogynecol J 17:326–329
Neuman M, Lavy Y (2007) Posterior intra-vaginal slingplasty for the treatment of vaginal apex prolapse: medium-term results of 140 operations with a novel procedure. Eur J Obstet Gynecol Reprod Biol DOI 10.1016/j.ejogrb.2006.07.035
Biertho I, Dallemagne B, Dewandre JM, Markiewicz S, Monami B, Wahlen C, Weerts J, Jehaes C (2004) Intravaginal slingplasty: short term results. Acta Chir Belg 104(6):700–704
Yildirim A, Basok EK, Gulpinar T, Gurbuz C, Zemheri E, Tokuc R (2005) Tissue reactions of 5 sling materials and tissue material detachment strength of 4 synthetic mesh materials in a rabbit model. J Urol 174(5):2037–2040
Siegel AL, Kim M, Goldstein M, Levey S, Ilbeigi P (2005) High incidence of vaginal mesh erosion using the intravaginal slingplasty sling. J Urol 174:1308–1311
Rane A, Lim YN, Withey G, Muller R (2004) Magnetic resonance imaging findings following three different vaginal vault prolapse repair procedure: a randomized study. Aust N Z J Obstet Gynaecol 44:135–139
Sivaslioglu AA, Unlubilgin E, Dolen I (2007) The multifilament polypropylene tape erosion trouble: tape structure vs surgical technique. Which one is the cause? Int Urogynecol J Pelvic Floor Dysfunct DOI 10.1007/s00192-007-0456-z
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luck, A.M., Steele, A.C., Leong, F.C. et al. Short-term efficacy and complications of posterior intravaginal slingplasty. Int Urogynecol J 19, 795–799 (2008). https://doi.org/10.1007/s00192-007-0527-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-007-0527-1