Abstract
Overactive bladder syndrome (OBS) is described as urinary urgency with or without incontinence, usually with increased daytime frequency and nocturia in the absence of another identifiable pathological process. Nowadays and despite other alternative therapies, the mainstay of OBS is still the pharmacological approach, mainly with anti-muscarinic drugs. To compare the efficacy of a 30-day solifenacin succinate (5 mg OD) treatment with or without previous medication with trospium chloride, a prostective open, two-arm, parallel group study was conducted for 5 weeks in 40 patients with OBS. The primary endpoint was patient self-assessment of improvement after 30 days of medication. Secondary endpoints included the reduction of the daily number of voids and urgency or involuntary leakage episodes. Adverse reactions and therapeutic stoppage were also evaluated. To be included in the trospium chloride treatment group, patients were required to have been treated with such drug for 1 to 6 months before the present study. Evaluation and efficacy assessment were accomplished using a 3-day bladder diary and an urgency severity scale (USS). Safety assessment was done by recording all the patients’ complaints after starting medication. A total of 40 patients were enrolled for this study, 19 without previous medication and 21 who had already tried trospium chloride. Two patients from the non-previous medication group were excluded. Globally, there was a statistically significant reduction for the USS (2.73→1.73), the daily number of voids (9.5→7.0), of urgency episodes (9.1→4.0) and of involuntary leakage episodes (3.6→1.0) over the 24 h. Six patients had no improvement, four from the previous trospium chloride group and two from the non-previous medication group. Three patients reported side effects, two cases of dry mouth and one case of constipation. One patient dropped out of the treatment due to an unspecified intolerance. Solifenacin succinate 5 mg seems to be effective concerning patients’ self-assessment of improvement and decrease in the mean number of daily voids, urgency episodes and incontinence episodes. This was reported both in patients who have already been medicated with trospium chloride and those who have never taken any kind of medication. Regarding side effects, solifenacin is quite well-tolerated in both groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overactive bladder syndrome (OBS) is described as urinary urgency with or without incontinence, usually with increased daytime frequency and nocturia in the absence of another identifiable pathological process [1]. It affects 16% to 22% of American and European adults with higher prevalence among elder people [2]. Although OBS can seriously jeopardize [3] women’s quality of life, only about 60% of these seek treatment and only 27% get it [2].
Nowadays and despite other alternative therapies, the mainstay of OBS is still the pharmacological approach. Several drugs have been tried with different success rates, but the optimal one is still to be developed. Among these drugs, anti-muscarinic drugs are the most often used, aiming to decrease involuntary detrusor contractility, which results in voiding frequency reduction and better bladder compliance.
In our daily practice, we usually choose trospium chloride (20 mg bid) as our first option because of its low cost when compared with other drugs. This is an anti-cholinergic that mainly has an anti-muscarinic activity, but also with ganglionar action. Trospium chloride has the advantage of not crossing the blood–brain barrier and therefore has very few metabolic and drug interactions—it is not metabolized by cytochrome P450 isozymes [4, 5]; only 15% has hepatic metabolization and 80% is eliminated by the kidneys as an unchanged drug. It seems to be as effective as oxybutynin and tolterodine with an important decrease in incontinence episodes and improvement in health-related quality of life [6]. Although it has a favorable safety profile and a 50–60% improvement rate [7], about 54% of its users complain of side effects (dry mouth, blurred vision, constipation), which are often a reason for discontinuation. This rate is in line with oxybutynin and is probably higher than with tolterodine [8, 9].
On the other hand, solifenacin succinate (5 mg and 10 mg doses) is a once daily new generation anti-muscarinic agent that has demonstrated a good efficacy and tolerability profile in several trials [10–12]. When compared to tolterodine, it seems to be more efficacious in decreasing urgency episodes, incontinence, urge incontinence and in increasing the voided volume showing a similar discontinuation rate with mild to moderate side effects.
To our knowledge, no trial has been done comparing solifenacin with trospium chloride. Furthermore, it still remains unproven if patients that drop out of treatment with this medication due to lack of efficacy or side effects will benefit from solifenacin succinate treatment. This trial was designed to test the efficacy of the primary use of solifenacin succinate (5 mg OD) after a 30-day treatment compared to its use after unsuccessful treatment with trospium chloride.
Materials and methods
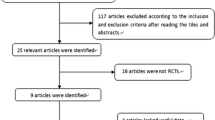
A prospective, open, two-arm, parallel group study was conducted for 30 days in 40 patients with OBS between January and April 2006. One of the arms included 19 patients without any previous medication for OBS and the other arm included 21 patients who had been previously unsuccessfully treated with trospium chloride.
All women were medicated with solifenacin succinate 5 mg OD.
The primary endpoint was patient self-assessment of improvement after 30 days of medication. Secondary endpoints included the reduction of the daily number of voids and urgency or involuntary leakage episodes. Adverse reactions and therapeutic stoppage were also evaluated.
The inclusion criteria for the study were: women aged over 18 with OBS symptoms for at least 3 months, an average of 8 or more voids per 24 h and 1 or more incontinence episode per 24 h. The exclusion criteria included: stress urinary incontinence or mixed incontinence with predominant stress incontinence, neurological disease, advance stage diabetes, previous pelvic irradiation, bladder lithiasis, previous pelvic malignant disease, glaucoma, urinary retention >200 ml, pelvic training in the previous month and pregnancy. To be included in the previous trospium chloride treatment group, patients were required to have been treated with such drug for 1 and 6 months before the present study and treatment stoppage due to lack of improvement or adverse reactions.
Evaluation and efficacy assessment were accomplished using a 3 day bladder diary, the first completed before treatment and the second during the last 3 days of treatment. An urgency severity scale (0—no urgency; 1—awareness of urgency but easily tolerated without interference in usual activity tasks; 2—enough urgency to interfere with usual tasks; 3—extreme urgency discomfort that abruptly stops all activity) was also used before and at the end of treatment. Concerning safety assessment, all the patients’ complaints after starting medication were recorded. Their severity was considered as mild if it did not led to treatment interruption or severe if it did.
The efficacy of solifenacin succinate was evaluated in both groups before and after treatment with paired t test for the USS score, urgency episodes, involuntary leakage episodes and number of daily voids. The t test was used to compare the treatment results in both groups. The rate of improvement with solifenacin succinate for the previous endpoints was assessed in both groups and classified in three classes: NI—no improvement; MI—mild improvement (<50% improvement) and GI—great improvement (≥50% or more improvement). The number of daily voids reduction endpoint was an exception, the comparison being made between reduction and no reduction.
Results
A total of 40 patients were enrolled for this study, 19 without previous medication and 21 with previous treatment with trospium chloride. Of these 21 patients, 5 dropped out of trospium chloride treatment because of adverse effects mainly dry mouth and constipation and the other 16 stopped because of the lack of improvement.
Two patients from the non-previous medication group were excluded from the improvement analyses due to inadequate completion of the bladder diary. They were, however, included for the adverse reactions analysis. There was one drop out during the study. A patient from the previous trospium chloride group stopped taking solifenacin on the third day because of unspecified intolerance. This patient was not considered for efficacy assessment, but was taken into consideration for safety analysis.
The two groups had very similar populations (Table 1), the mean age being 60.6 in group A and 58.2 for group B (SD 12.5 vs 12.9). The mean BMI was 28.2 in group A vs 29.8 in group B. Concerning the baseline characteristics, the total population had an average of 9.5 voids/24h, 9.1 urgency episodes/24 h and 3.6 incontinence episodes/24 h. Regarding the USS score, the average was 2.73 with nearly 70% of the patients with grade 3.
Efficacy
The primary endpoint was the patient self-assessment of improvement after solifenacin treatment in both groups. This was assessed comparing the USS score (which was in average 2.73 before treatment and 1.73 after treatment) resulting in a statistically significant improvement (p < 0.001). From the total 38 patients, 6 (18%) referred no improvement (2 from group A and 4 from group B). No statistically significant difference was seen between group A (2.71→1.23) and group B (2.75→1.2). Overall, 6 patients (16%) had no improvement, 5 (13.5%) had mild improvement and 26 (69.5%) had great improvement (Fig. 1).
On the secondary endpoints (reduction in the number of urgency, the number of involuntary leakage episodes and the number of voids over the 24 h), our results showed a statistically significant (p < 0.001) decrease in all these issues in both groups. Once again, no statistically significant difference was seen between group A and group B. Overall, 6 patients (16%) had no improvement in the number of involuntary leakage episodes during the 24 h, 4 (11%) had mild improvement and 27 (73%) had great improvement (Fig. 2). Regarding the number of daily urgency episodes, the bladder diary results showed no improvement in 5 patients (13.5%), a mild reduction in 10 patients (27%) and a great reduction in 22 patients (59%) (Fig. 3).
Safety
Overall, three patients reported adverse effects, two from group A and one from group B. The side effects reported were two cases of dry mouth and one case of constipation, none of them being severe enough to stop treatment. However, as it has already been said, there was one patient from the previous trospium chloride use group that dropped out of treatment on the third day because of an unspecified intolerance with the medication, which ceased with the treatment stoppage. None of these patients from group B had dropped out of trospium chloride for intolerance, but for lack of efficacy.
Discussion
Solifenacin is a once daily oral anti-muscarinic that seems to be effective for the treatment of OBS. Several studies [10, 11], have previously reported its efficacy concerning the reduction of urgency and increase of functional bladder capacity. This leads to a decrease in the number of daily voids and incontinence episodes and also to an improvement in women’s quality of life. When directly compared with older anti-cholinergic medication like tolterodine, solifenacin seems to be more efficacious. However, to our knowledge, it remains unproven if patients previously medicated with trospium chloride without improvement or who had to drop out of this medication for side effects will benefit with this treatment. Our study was designed to evaluate this issue and we used two different instruments for the efficacy assessment, a subjective one—the USS score and an objective one—a 3-day bladder diary. Our results showed a statistically significant improvement concerning both the subjective and the objective endpoints when the groups were compared before and after 30 days of treatment. Although there was a higher rate of patients from the previous trospium chloride group without improvement, this result was not statistically significant.
Taking into consideration that both substances act as an anti-cholinergic, we would expect better results from the no previous medication group. We don’t know the reason for this, although several reasons may help explain it. First of all, both of them are anti-cholinergic but their target of action is different. Second, trospium chloride doses may vary and a twice daily scheme may lead to a worse compliant behavior, which leads to worse improvement rates. Third, minor side effects not reported by the patients might also lead to a worse compliant behavior. Other studies would be required to assess these reasons.
Concerning side effects, solifenacin succinate was quite well-tolerated with mainly minor complains, which is consistent with the literature. The results presented here show a better tolerance profile favoring solifenacin succinate when compared to trospium chloride data previously reported. However, one of our patients from the previous trospium chloride treatment group dropped out of solifenacin succinate with complaints we cannot justify as being part of the properties of this substance. No statistically significant differences were seen between the two groups and once again, intolerance with trospium chloride was not predictive of intolerance with solifenacin succinate.
On our daily practice and according to the low social economic level of our population, we still start medication with trospium chloride because of its low cost. Whenever there is intolerance or lack of efficacy, we change to a second line drug, solifenacin succinate being one of the options.
Conclusion
Although our study used a small sample, the results showed that solifenacin succinate 5 mg OD seems to be effective for the treatment of OBS. This is reflected in patients self-assessment of improvement and in the decrease of the mean daily voids number, urgency episodes and incontinence episodes both in patients who have already been medicated with trospium chloride and those who have never taken any kind of medication. Regarding side effects, solifenacin succinate is quite well-tolerated. Previous treatment with trospium chloride was not predictive of a worse response or poorer tolerance.
References
Milsom I, Abrams P, Cardozo L et al (2001) How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int 87:760–766
Abrams P, Cardozo L, Fall M et al (2002) The standardization of terminology of lower urinary tract function: report from the Standardization Sub-committee of the International Continence Society. Neurourol Urodyn 21:167–178
Coyne KS, Payne C, Bhattacharyya SK et al (2004) The impact of urinary urgency and frequency on health-related quality of life in overactive bladder: results from a national community survey. Value Health 7:455–463
Zinner NR (2005) Trospium chloride: an anticholinergic quaternary ammonium compound for the treatment of overactive bladder. Expert Opin Pharmacother 6:1409–1420
Rovner ES (2004) Trospium chloride in the management of overactive bladder. Drugs 64:2433–2446
Singh-Franco D, Machado C, Tuteja S, Zapantis A (2005) Trospium chloride for the treatment of overactive bladder with urge incontinence. Clin Ther 27:511–530
Andersson KE et al (2002) Pharmacological treatment of urinary incontinence. In: Abrams P, Khoury S, Wein A (eds) Incontinence. Second International Consultation on Incontinence. Plymbridge Distributors, Plymouth, United Kingdom, pp 489–511
Malone-Lee J, Shaffu B, Anand C, Powell C (2001) Tolterodine: superior tolerability than and comparable efficacy to oxybutynin in individuals 50 years old or older with overactive bladder: a randomized controlled trial. J Urol 165:1452–1456
Abrams P, Malone-Lee J, Jacquetin B et al (2002) Twelve-month treatment of overactive bladder: efficacy and tolerability of tolterodine. Drugs Aging 18:551–560
Cardozo L, Lisec M, Millard R et al (2004) Randomized, double-blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. J Urol 172:1919–1924
Chapple CR, Rechberger T, Al-Shukri S et al (2004) Randomized, double-blind placebo- and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU Int 93:303–310
Chapple CR, Martinez-Garcia R, Selvaggi L et al (2005) A comparison of the efficacy and tolerability of solifenacin succinate and extended release tolterodine at treating overactive bladder syndrome: results of the STAR trial. Eur Urol 48:464–470
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Metello, J., Nogueira, B., Torgal, M. et al. Comparison of the efficacy and tolerability of solifenacin succinate with or without previous use of trospium chloride. Int Urogynecol J 18, 1021–1025 (2007). https://doi.org/10.1007/s00192-006-0271-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-006-0271-y