Abstract
The aim of the study was to measure pelvic floor muscle function in continent and incontinent nulliparous pregnant women. The study group consisted of 103 nulliparous pregnant women at 20 weeks of pregnancy. Women reporting urinary incontinence once per week or more during the previous month were classified as incontinent. Function was measured by vaginal squeeze pressure (muscle strength) and increment in thickness of the superficial pelvic floor muscles (urogenital diaphragm) assessed by perineal ultrasound. Seventy-one women were classified as continent and 32 women as incontinent. Continent women had statistically significantly higher maximal vaginal squeeze pressure and increment in muscle thickness when compared with incontinent women. There was a strong correlation between measurements of vaginal squeeze pressure and perineal ultrasound measurements of increment in muscle thickness. This study demonstrates statistically significant differences in pelvic floor muscle function measured by strength and thickness in continent compared with incontinent nulliparous pregnant women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary incontinence is a common symptom during pregnancy, with prevalence rates varying between 20–67% [1, 2]. In some women urinary incontinence diminishes after delivery; in others the problem persists [3, 4]. Strategies to prevent urinary incontinence during pregnancy may be important in preventing urinary incontinence later in life. Aims of prevention would be to remove the cause of incontinence or to treat dysfunction early to stop progression. To be able to do so, one needs knowledge about risk factors and anatomical differences between continent and incontinent pregnant women.
The pelvic floor muscles play an important role in the maintenance of urinary continence [5, 6, 7, 8]. The pelvic floor muscles consist of two muscular layers, the pelvic diaphragm and the urogenital diaphragm (the perineal membrane with its striated muscles and the external genital muscles) [6]. The muscular layers form a structural support; the pelvic floor muscle volume influences the anatomical location of the pelvic organs, and a fast and strong contraction of the pelvic floor muscles ensures continence during abrupt increase in abdominal pressure [5, 6]. While it is assumed that reduced pelvic floor muscle volume and strength are critical factors in the pathogenesis of urinary incontinence, there is still a lack of studies describing pelvic floor muscle volume and strength in continent and incontinent women.
Several techniques have been used to evaluate pelvic floor muscle strength [9]. Hahn et al. [10] compared pelvic floor muscle strength of continent and incontinent women as measured by vaginal squeeze pressure, digital palpation and vaginal cones. They found statistically significantly stronger pelvic floor muscles in the continent group. However, they also concluded that all three measuring techniques had limitations.
Ultrasound can visualize anatomical alterations related to the urethrovesical junction associated with stress urinary incontinence [11]. Pelvic floor muscle function, often referred to as levator ani function, has been measured indirectly by ultrasound assessment of, e.g., bladder neck elevation during contraction of the pelvic floor muscles [12, 13, 14, 15, 16]. However, this is one of many, indirect measures of the function of one part of the pelvic floor muscles. Three-dimensional ultrasound has recently been introduced as a method to study the complex anatomy of the pelvic floor [17, 18] and may be a method that can give new information concerning the role of the pelvic floor muscles. We have found only one study where pelvic floor muscle volume (thickness) has been measured directly and related to continence status [19]. Bernstein [19] used perineal ultrasound and found that pelvic floor muscle thickness was lower in a group of incontinent women than in a group of continent women.
It has been recognized that vaginal delivery may cause damage to the pelvic floor muscles, fascias and nerves [8]; thus, the inclusion of parous women may have influenced the results in the studies by Bernstein [19] and Hahn et al. [10].
At present there is a lack of evidence to support a hypothesis that pelvic floor muscle strength and thickness are related to continence status. A possible correlation between pelvic floor muscle strength and muscle thickness has not been studied.
The aim of this study was to measure pelvic floor muscle function by using methods to assess muscle strength and thickness in continent and incontinent nulliparous pregnant women. In addition, we wanted to study a possible correlation between measurements of pelvic floor muscle strength and thickness.
Materials and methods
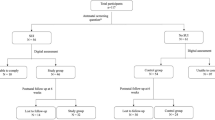
The study group consisted of 103 out of 301 nulliparous women at 18–20 weeks of pregnancy, recruited to a randomized controlled trial evaluating pelvic floor muscle training [20]. The women came from a non-selected population from a geographically well-defined area consisting of four municipalities surrounding and including the city of Trondheim, and were included from October 1998 to May 2000. Women were eligible for the trial if they were nulliparous, 18 years or more and had a singleton live fetus at a routine ultrasound scan at 18 weeks of pregnancy. Exclusion criteria were pregnancy complications, high risk for pre-term labor, pain during pelvic floor muscle contractions, ongoing urinary tract infection, or diseases that could interfere with participation.
The 103 participants in the present study consisted of all the women in the trial that were included on Mondays, which for practical reasons was the only day when the pelvic floor ultrasound measurement could be done. Thus, all women who could meet for assessments in the trial on a Monday were also included in the present study. The women were examined with perineal ultrasound and pelvic floor muscle strength assessments before they were randomized into two groups in the trial. All women gave a written informed consent and approval was obtained from the local medical research ethics committee.
Classification of continent/incontinent: structured interview
One investigator (SM) performed all the interviews. Information concerning continence status was collected after the assessments of pelvic floor muscle thickness and strength. All subjects were asked about symptoms of urinary incontinence and background information. Questions to classify continence status were: “Do you leak urine more than once per day, once per day, more than once per week, once per week, less than once per week?” Women reporting urinary incontinence once per week or more during the last month were classified as incontinent. The type of urinary incontinence was addressed with additional questions. Body mass index, information about participation in physical activities and pelvic floor muscle exercises were registered.
Pelvic floor muscle function
Before the assessments of pelvic floor muscle strength and thickness were performed, all women were instructed in pelvic floor anatomy and how to contract the pelvic floor muscles correctly. The principal investigator (SM) used vaginal palpation and observation to examine the ability to perform pelvic floor muscle contraction [21, 22]. The women were in a supine position with straight legs. One finger was used for palpation. No observable synergistic contractions of hip adductors and gluteal muscles, or pelvic tilt, were accepted as correct contractions.
Measurement of pelvic floor muscle strength
Muscle strength was measured by one investigator (SM) by using a vaginal balloon catheter (balloon size 6.7×1.7 cm) connected to a pressure transducer (Camtech Ltd. 1300 Sandvika, Norway). Vaginal squeeze pressure (cm H2O) during pelvic floor muscle contractions was measured. The middle of the balloon catheter was positioned approximately 3.5 cm inside the introitus vagina [22]. Only contractions with observed inward movement of the balloon catheter were accepted. The method was found to be reliable and valid in a previous study [22].
Measurement of thickness of the superficial pelvic floor muscle
Perineal ultrasound was used to measure the thickness of the urogenital diaphragm, the muscular layer of the pelvic floor situated caudal to the pelvic diaphragm and anterior to the anorectum [6]. A Vingmed CFM 800 ultrasound device with a 7.5-MHz vaginal probe was used, and one investigator (SM) performed all examinations. Women were examined in a supine position with 45° hip flexion and slight abduction. The long axis of the transducer was held approximately parallel to the examination bench and placed in a parasagittal section on the perineum (Fig. 1a), just to the right of the vaginal introitus, approximately midway between the urethra and the posterior commisur of the introitus (Fig. 1b). With the transducer in this position the plane was moved parallel to the left until the vaginal wall was visualized and then about 1 cm to the right. The scanning plane was further secured by visualizing the pubic bone as a landmark to the right on the ultrasound screen; a vertical reference line then could be drawn in the middle of the screen (Fig. 2). The pelvic floor muscles (urogenital diaphragm) then could be identified as a hypoechoic structure beneath the subcutaneous fascia 3–6 mm below the skin surface (Figs. 2 and 3). Slight movements of the transducer were often necessary to improve the delineation of the muscle towards the surrounding tissue. Further assurance of the examination plane was done by asking the woman to relax the pelvic floor muscles and then perform maximum contraction. Muscle movement during contraction was visualized dynamically and muscle thickness was measured in millimeters both during relaxation and contraction (Fig. 3). All measurements were performed as triple measurements, and values were given as mean and 95% confidence interval of their triple measurements. Pelvic floor muscle increment is given as the difference between measurements of pelvic floor muscle thickness during relaxation and contraction.
The ultrasound measurement method was tested for intra-observer reliability (Intraclass Correlation Coefficient [ICC] one-way random effect model, average measure intraclass correlation = 0.9841) ( n =50). To test direct applicability in clinical practice, we also tested inter-observer reliability between two investigators with different experience with the method, in 23 women (ICC two-way random effect model, average measure intraclass correlation = 0.7123).
Statistical methods
Except for frequencies, background variables are given as mean and 95% confidence interval (95% CI). Pelvic floor muscle thickness and strength are reported as Mean values and 95% CI. Correlation was tested by the Pearson correlation coefficient (r), and the two-sample t-test for equality of means was used to test the differences between independent groups of different sample sizes. P values <0.05 were considered significant. Intra- and inter-observer reliability were tested by using the Intraclass Correlation Coefficient (SPSS; ICC one-way and two-way random effect model).
Results
Seventy-one women were classified as continent and 32 women as incontinent. After instruction, 102 of 103 women were able to perform pelvic floor muscle contractions correctly. Background variables were similar in the groups of continent and incontinent women (Table 1).
The mean pelvic floor muscle strength measured as vaginal squeeze pressure among continent women was 39.5 cm H2O (95% CI; 35.7–43.4), whereas incontinent women had a mean vaginal squeeze pressure of 32.0 cm H2O (95% CI; 27.7–36.3). This difference in maximal pelvic floor muscle strength was statistically significant ( P =0.010).
In the group of continent women the superficial pelvic floor muscles was statistically significantly thicker both during relaxation ( P =0.018) and contraction ( P =0.006) than in the group of incontinent women (Table 2). Continent women also had statistically significantly ( P =0.021) higher mean increments in muscle thickness (Table 2).
The correlation ( r =0.703) between measurements of maximal pelvic floor muscle strength and perineal ultrasound measurements of increment in muscle thickness is shown in Fig. 4.
Discussion
The statistically significant difference in pelvic floor muscle strength and thickness of the urogenital diaphragm between continent and incontinent nulliparous women demonstrated in this study supports the hypothesis that pelvic floor muscle strength and thickness are related to continence status. No previous study has reported on both thickness and strength of the pelvic floor muscles and correlation between the two assessment methods. One other research group has measured pelvic floor muscle thickness with ultrasound [19]. Bernstein [19] found higher pelvic floor muscle thickness at rest and during contraction, but did not find significant differences in muscle increment between groups of continent and incontinent women.
We found a mean resting muscle thickness of 6.3 mm in incontinent women and 8.2 mm in continent women. Bernstein [19] reported higher values. The discrepancy in muscle thickness between the two studies may be due to differences in the study populations, but the differences may also be due to different measurement techniques. Identification and measurement of the muscles in the pelvic floor is not easy to perform. We decided to measure the pelvic floor muscles situated around the distal part of the urethra because activity of these muscles is supposed to compress the urethra distally, causing the urethral pressure rise that precedes and exceeds the rise in abdominal pressure during a cough [6]. Thus, we measured the thickness of the muscular layer lateral to the vagina, 3–6 mm below the skin surface. According to anatomical studies and magnetic resonance images, this corresponds to the urogenital diaphragm, situated caudal to the pelvic diaphragm and anterior to the anorectum [6, 23, 24, 25, 26] (Figs. 1, 2 and 3). According to DeLancey [6], contraction of the muscles in the urogenital diaphragm would press upon the anterior aspect of the urethral lumen and thereby compress it. The pelvic diaphragm including m. levator ani is more deeply situated, and has a different muscle fiber direction and function than the muscular layer measured in our study. M. levator ani appears to be iso- or hyperechoic on the ultrasound screen. We found it difficult to do precise assessments of the thickness of the m. levator ani by using the same perineal ultrasound application as we used to measure the urogenital diaphragm. Thus, our measurements of the thickness of the pelvic floor muscles in the present study were not meant to include the thickness of both the muscular layers.
The anatomy of the pelvic floor is described differently in various papers and textbooks, and ultrasound of the muscular layers is difficult to perform. We hoped to find a method to visualize the dynamic activity and the thickness of the superficial pelvic floor muscles that could be implemented in clinical practice. The intra-tester reliability in our study was high (ICC 0.98), but the inter-tester reliability was not as good (ICC 0.71). Obviously, there is a learning curve in the assessment of muscle thickness with perineal ultrasound. Thus, there is a need for detailed anatomical knowledge and a fair amount of practical training before the method can be used in a clinical setting.
Ultrasonography for visualization of the pelvic floor muscles should be further explored. It enables dynamic functional studies, visualization of the bladder neck, the proximal urethra, the pelvic floor muscles and their relationship to the pubic symphysis and the changes during muscle contraction. Methods to measure distances and angles of the urethrovesical unit have been described [27]. Dietz et al. [16] used perineal ultrasound to visualize bladder neck elevation following levator activity, to provide visual biofeedback for women unable to perform levator contractions correctly. In addition, perineal ultrasound has been used to investigate the effects of vaginal delivery and caesarian section on bladder neck mobility and genuine stress incontinence [14, 28]. In future studies, the use of three-dimensional ultrasound should be explored to give additional information of the complex anatomy of the pelvic floor.
We found statistically significantly higher pelvic floor muscle strength in continent compared with incontinent nulliparous women at 20 weeks of pregnancy. This result corresponds well to the findings of Hahn et al. [10]. However, there are differences in the measured values reported in the two studies, probably because of different measurement methods and study populations. There have been discussions related to the validity of squeeze pressure measurements as an indicator of pelvic floor muscle strength [9, 10]. Squeeze pressure aims to measure muscle strength, including both activated motor units and the effect of muscle volume. Vaginal squeeze pressure measurement has several problems, the most serious one being that both straining and a correct contraction can give equal increases in pressure [22]. Hence, only contractions with observed simultaneous inward movement of the perineum was considered to give valid measurements in the present study [21, 22].
In the present study, self reported symptoms of urinary incontinence were used to classify continent and incontinent participants. Since we studied healthy pregnant women, we found it important to use diagnostic tests and outcome measurements causing minimal discomfort to the participants. We refrained from using pad tests with standardized bladder volume in order to avoid inducing urinary tract infections among the pregnant women. Lagro-Janssen et al. [29] concluded that urodynamics are unnecessary in most women presenting with urinary incontinence in general practice, but other studies have focused on the need for urodynamic assessment in making a diagnosis and formulating a treatment plan [30, 31].
We found a strong correlation between muscle thickness and muscle strength. In general, there is a strong correlation between the cross-sectional area of a muscle and its absolute strength capabilities [32, 33]. The correlation between pressure and thickness measurements in this study may indicate that both methods are valid and reliable methods of assessing pelvic floor muscles. The same examiner collected all data, and this fact could introduce a possible bias in the study. However, the collection of data regarding continence status was done after the measurements of muscle strength and thickness.
One important question is whether low pelvic floor muscle strength and thickness should be considered to be risk factors for the development of urinary incontinence. If this were so, the clinical implication of the present study could be to assess muscle thickness and strength in early pregnancy, and to suggest pelvic floor muscle strength training in women with low values. However, the structural support that is important to enable continence is most likely provided by an integrated action of fasciae and muscles under neural control [5], and measurements of thickness or pressure may be a too simplistic model. Nevertheless, the results of the present study indicate that the pelvic floor muscle volume and strength plays an important role in maintaining urinary continence.
Conclusions
This study demonstrates statistically significant differences in pelvic floor muscle function measured by muscle strength and thickness in continent compared with incontinent nulliparous pregnant women. There was a strong correlation between measurements of pelvic floor muscle strength and thickness of the superficial pelvic floor muscles.
References
Viktrup L, Lose G, Rolf M, Barfoed K (1993) The frequency of urinary symptoms during pregnancy and puerperium in the primipara. Int Urogynecol J 4:27–30
Burgio KL, Locher JL, Zyczynski H, Hardin JM, Singh K (1996) Urinary incontinence during pregnancy in a racially mixed sample: characteristics and predisposing factors. Int Urogynecol J 7:69–70
Mørkved S, Bø K (1999) Prevalence of urinary incontinence during pregnancy and postpartum. Int Urogyn J 10:394–398
Viktrup L (2002) The risk of lower urinary tract symptoms five years after the first delivery. Neurourol Urodyn 21:2–29
De Lancey JOL (1996) Stress urinary incontinence: where are we now, where should we go? Am J Obstet Gynecol 175:311–319
DeLancey JOL (1988) Structural aspects of the extrinsic continence mechanism. Obstet Gynecol 72:296–300
Koelbl H, Strassegger H, Riss PA, Gruber H (1989) Morphologic and functional aspects of pelvic floor muscles in patients with pelvic relaxation and genuine stress incontinence. Obstet Gynecol 74:789–795
Sultan AH, Kamm MA, Bartram CI, Hudson CN (1994) Perineal damage at delivery. Contemp Rev Obstet Gynaecol 6:18–24
Peschers UM, Gingelmaier A, Jundt K, Leib B, Dimpfl T (2001) Evaluation of pelvic floor muscle strength using four different techniques. Int Urogynecol J 12:27–30
Hahn I, Milsom I, Ohlsson BL, Ekelund P, Uhlemann C, Fall M (1993) Pelvic floor training for genuine stress urinary incontinence. Evaluation and long-term results (dissertation). The University of Gothenburg, Gothenburg, Sweden
Petri E, Koelbl H, Schaer G (1999) What is the place of ultrasound in urogynecology? A written panel. Int Urogynecol J 10:262–273
Kohorn EI, Sciosa AL, Jeanty P (1986) Ultrasound cystourethrography by perineal scanning for the assessment of female stress urinary incontinence. Obstet Gynecol 68:269–272
Schaer GN, Koechli B, Schuessler B, Haller U (1995) Perineal ultrasound for evaluating the bladder neck in urinary stress incontinence. Obstet Gynecol 85:220–224
Peschers UM, Schaer G, DeLancey JOL, Schuessler B (1997) Levator function before and after childbirth. Br J Obstet Gynaecol 104:1004–1008
Meyer S, Bachelard O, DeGrandi (1998) Do bladder neck mobility and urethral sphincter function differ during pregnancy compared with during the non-pregnant state? Int Urogynecol J 9:397–404
Dietz HP, Wilson PH, Clarke B (2001) The use of perineal ultrasound to quantify levator activity and teach pelvic floor muscle exercises. Int Urogynecol J 12:166–169
Wisser J, Schaer G, Kurmanavicius J, Huch R, Huch A (1999) New approach to assess obstetrical trauma to the pelvic floor with 3-D ultrasound. Ultraschall Med 20:15–18
Ochsenbein N, Kurmanavicius J, Huch R, Huch A, Wisser J (2001) Volume sonography of the pelvic floor in nulliparous women and after elective cesarean section. Acta Obstet Gynecol Scand 80:611–615
Bernstein IT (1997) The pelvic floor muscles: muscle thickness in healthy and urinary-incontinent women measured by perineal ultrasonography with reference to the effect of pelvic floor training. Estrogen receptor studies (dissertation). Neurourol Urodyn 16:237–275
Mørkved S, Bø K, Schei B, Salvesen KÅ (2003) Pelvic floor muscle training during pregnancy to prevent urinary incontinence—a single blind randomized controlled trial. Obstetrics & Gynecology 101:313–319
Kegel AH (1956) Stress incontinence of urine in women: physiological treatment. J Int Coll Surg 25:487–499
Bø K, Hagen RH, Kvarstein B, Larsen S (1990) Pelvic floor muscle exercise for the treatment of female stress urinary incontinence II. Validity of vaginal pressure measurements of pelvic floor muscle strength. The necessity of supplementary methods for control of correct contraction. Neurourol Urodyn 9:479–487
Nichols DH, Milley PS (1971) The relationship between the pubo-urethral ligaments and the urogenital diaphragm in the human female. Anat Rec 170:281
Oelrich TM (1983) The striated urogenital sphincter muscle in the female. The anatomical record 205:223–232
Tan IL, Stoker J, Zwamborn AW, Entius KAC, Calame JJ, Lameris JS (1998) Female pelvic floor: endovaginal MR imaging of normal anatomy. Radiology 206:777–783
Stoker J, Halligan S, Bartram CI (2001) Pelvic floor imaging. Radiology 218:621–641
Schaer GN, Koechli B, Schuessler B, Haller U (1996) Perineal ultrasound: determination of reliable examination procedures. Ultrasound Obstet Gynecol 7:347–352
Demirci F, Ozden S, Alpay Z, Demirci ET, Ayas S (2001) The effects of vaginal delivery and cesarean section on bladder neck mobility and stress urinary incontinence. Int Urogynecol J 12:129–133
Lagro-Janssen ALM, Debruyne FMJ, Van Weel C (1991) Value of patient’s case history in diagnosing urinary incontinence in general practice. Br J Urol 67:569–572
Clarke B (1997) The role of urodynamic assessment in the diagnosis of lower urinary tract disorders. Int Urogynecol J 8:196–200
Cundiff G, Harris RL, Coates KW, Bump RC (1997) Clinical predictors of urinary incontinence in women. Am J Obstet Gynecol 177:262–267
DiNubile NA (1991) Strength training. Clin Sports Med 10:33–62
Komi PV (1992) Strength and power in sport. Blackwell scientific publications
Acknowledgements
We want to thank Nancy Lea Eik-Nes for the English revision of the manuscript. The work was founded by The Norwegian Fund for Postgraduate Training in Physiotherapy and Norwegian Women’s Public Health Association.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial Comment: This study evaluated pelvic floor muscle function in 103 nulliparous continent and incontinent women at 18–20 weeks gestation. Pelvic floor muscle strength was assessed by measuring vaginal squeeze pressure, and thickness of the urogenital diaphragm during both relaxation and contraction was measured using perineal ultrasound. The authors found a statistically significant higher vaginal squeeze pressure and higher mean increment in muscle thickness in the continent compared with incontinent group as well as a strong correlation between pelvic floor muscle strength and increment in thickness. Although describing several benefits of ultrasonography in assessing pelvic floor muscles, the authors did acknowledge the difficulty in identifying and measuring these muscles, and the learning curve involved with perineal ultrasound. Another limitation was the subjective classification of continence status based on self-reported symptoms. The implication of low pelvic floor muscle strength and thickness as risk factors for the development of urinary incontinence is beyond the scope of this study.
Rights and permissions
About this article
Cite this article
Mørkved, S., Salvesen, K.Å., Bø, K. et al. Pelvic floor muscle strength and thickness in continent and incontinent nulliparous pregnant women. Int Urogynecol J 15, 384–390 (2004). https://doi.org/10.1007/s00192-004-1194-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-004-1194-0