Abstract
This paper is about adding magnetic and ethanol to explore the accuracy of electrochemical discharge machining processing quartz glass. The tool electrode is tungsten carbide rod, the auxiliary electrode is platinum, the power source uses square wave pulse voltage, the KOH electrolyte is added with ethanol, and the tool is added with 3 T magnetic force. The experimental results show that ethanol can stabilize the square wave power supply wave shape, which can reduce the contact angle between the electrode and the bubble. The low contact angle electrolyte can increase the wettability of the tool electrode and improve the electrochemical processing stability. Therefore, under the action of ethanol and a magnetic field, the processing result can be improved, so that the generated bubbles are reduced and the film gets thinned. When the voltage frequency is higher, the film formation thickness will decrease, and the magnetic force and voltage will induce the magnetohydrodynamics of the electrolyte, which will make the electrolyte and bubble flow around the electrode relatively stable, the circumference around the aperture is flat, and the roundness is obviously improved. The overall improvement in the taper of the machined hole is increased by about 30%, and the amount of undercut of the hole is reduced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In microelectromechanical systems (MEMS), because of its unique optical properties, quartz can also be used as a sensor for motion and pressure. Therefore, quartz has an important position for electronic components, but it is easy to cause residual stress and damage due to the processing method of polishing. Due to the specific functions of different components, it is necessary to fabricate specific microstructures such as micro-hole and grooves on the quartz material. Due to its hard and brittle characteristics, it is difficult to use conventional processing methods. Therefore, in this paper, the electrochemical processing method is used to explore the way of processing quartz materials and related methods to improve processing efficiency.
It is found in the relevant literature that the frequency and density of high-temperature sparks are the key to determining whether the overall energy can overcome the melting point and high hardness of engineering ceramics. It is found in the relevant literature that the frequency and density of high-temperature sparks is the key to determining whether the overall energy can overcome the melting point and high hardness of engineering ceramics. Based on the study of the conditions of electrochemical discharge phenomena, the empirical equation between critical voltage and current is found [1]. In electrochemical discharge machining, the bubble is sometimes completely blocked, and the current is completely reduced to zero. Therefore, a model of the discharge in the gas is proposed to explain the experimental data [2]. In the same hydrochloric acid (HCl) electrolyte, the cathode tip produces a discharge phenomenon for tantalum and metal [3]. In the literature, the tip of the electrode was used to observe the bubble reaction of ECDM, which shows the mutual conversion reaction of the observed current during the voltage rise [4]. Using a stochastic model combined with the transformation of percolation theory, a more complete model is proposed to derive the bubble coating process. The relationship between the bubble and current density is discussed, and the theoretical model is established. It is proved by theory that the wettability of the electrode will improve the stability of the thickness of the film [5, 6]. It has been studied to use the gravity-feed machining method to drill glass, and it is proposed that there are different hole shapes at different processing depths and voltages [7]. When the surface roughness of the tool electrode is higher, the growth of the gas film is increased, the contact angle is also increased, and the wettability is lowered [8]. If the energy of using ECDM is processed for ceramic materials, there are cases where the processing efficiency is rather low and the surface profile is poor [9]. If the glass is processed by ECDM and the phenomenon of spark reaction and chemical etching during processing is analyzed, the relevant processing conditions are proposed [10, 11]. The cylindrical electrode is electrically processed into a spherical electrode, and the quartz glass is processed by gravity-feed processing, which can reduce the processing time and reduce the aperture [12]. The addition of a magnetic field in the experiment can cause MHD convection, which can effectively enhance the electrolyte circulation in the micro-hole, thereby contributing to an increase in the overall entrance diameter of 23.8% [13, 14]. The experiment of adding ethanol to the KOH electrolyte can reduce the contact angle between the electrode and the bubble, and the formation of the gas film will become thinner [15]. Based on the above-mentioned related literatures, this paper adds magnetic and ethanol to ECDM processing glass, uses high-speed camera to capture the film phenomenon of electrochemical machining electrodes, and discusses the influence of experimental processing parameters.
1.1 Proposed methods
The electrochemical reaction is generated on the contact surface between the electrode and the electrolyte, and as the applied voltage increases, the hydrogen bubbles originally generated on the cathode form a bubble film. This bubble film blocks the original current path between the two poles, causing a current interruption. When the voltage continues to increase, the electric field strength between the two poles will also increase. Under the action of the electric field force, the charged ions and the molecules will collide with each other, breaking through the bubble insulation formed on the tool electrode due to the bubble film. Layers, and the formation of conductive channels, the phenomenon of electrochemical discharge, will generate a discharge spark on the tool electrode and electrolyte liquid surface, when the distance between the workpiece and the tool electrode is close enough, the workpiece can be thermally melted and remove the material. In this paper, due to the use of alkaline electrolyte, it is known from the literature [43] that the auxiliary electrode (Pt) will only dissociate into Pt2+ under acidic solution, so it will not be dissociated during the ECDM reaction. In the reaction system, the anode usually plays the role of an auxiliary electrode. If KOH is used as the electrolyte for electrochemical discharge processing, the reaction on the anode is mainly the oxidation reaction of hydroxide ions, producing water and oxygen. The main reaction of the cathode is that water molecules are subjected to electrolysis to generate hydrogen gas, and hydrogen bubbles are coated on the surface of the cathode.
The auxiliary electrode reaction formula is as follows:
The cathodic reaction formula is as follows:
Quartz glass, due to its special crystal structure and chemical composition, generally has a coordination effect with a ruthenium atom in an environment having a hydroxyl group (OH)–. Its overall reaction formula is as follows:
However, in the electrochemical discharge machining, the temperature of the electrolyte rises due to the continuous electrolysis reaction, and the high temperature generated causes the removal rate of the material to change, which can be determined by the relationship between the chemical reaction rate constant (KA) and the temperature. The relationship is as follows:
Where A is frequency factor i.e. the frequency of molecular collisions, E is activation energy i.e. the energy required to generate an activator for the reactive particles, R is gas constant, and T is absolute temperature (K).
1.2 Experimental setup
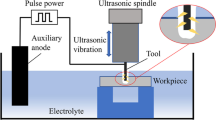
In this experiment, potassium hydroxide (KOH) was used as the electrolyte, the auxiliary electrode was platinum, the cathode was a tungsten carbide rod (ψ100 μm), and the workpiece was quartz glass. The power supply is a square wave pulse DC voltage with a pulse frequency between 1 and 500 Hz, Duty 20 and 50%. The computer software controls the feeding position of the tool. During the experiment, the electrolyte is added with 6.5 wt% ethanol, and the magnetic force of 3 T is added to the tool to investigate the influence of magnetic force on the quality and stability of the film and the processing result. The bubble generation was taken with a high-speed camera, the diameter was measured using a metallographic microscope, and the film thickness of the electrode tip and the taper of the hole were measured by image software analysis.
The Lorentz force is the force of a charged particle moving in an electromagnetic field. When the charged particle moves relative to the magnetic field, the particle is subjected to the Lorentz force, and because of the Lorentz force, the direction of the Lorentz force is \( {\overset{\rightharpoonup }{F}}_{\mathrm{L}}=q\overset{\rightharpoonup }{v}\times \overset{\rightharpoonup }{B} \) perpendicular to the direction of the velocity, where \( {\overset{\rightharpoonup }{F}}_{\mathrm{L}} \) is the Lorentz force, q is the charge of the ion, \( \overset{\rightharpoonup }{B} \) is the flux density, and \( \overset{\rightharpoonup }{v} \) is the velocity of the ion. According to Fleming’s left-hand rule, under the electromagnetic effect, Lorentz force is generated near the cathode electrode and will affect the flow of bubbles near the cathode electrode. During the processing, it can be obtained from the experimental diagram of Fig. 1. During the electrochemical discharge machining, the ions in the surrounding electrolyte are affected by the magnetic field and the electric field to generate Lorentz force, and the ions will generate circular motion. Around the electrode, the flow field around the electrode will also produce the same motion, so the bubble will have a circular motion with the flow field, which will affect the processing effect.
2 Results and discussion
2.1 Effect of frequency on film thickness
The voltage waveform used in this experiment is a square wave. The frequency determines the length of a single discharge time and also determines the size of the gas film. It is shown in Fig. 2 that at the same voltage, at a frequency of 1 Hz, the film radius at the tip of the electrode is the largest, and as the frequency increases, the thickness of the generated film decreases. In electrochemical discharge machining, however, the applied voltage must exceed the critical voltage to generate a spark discharge. The voltage is too small to be processed. If the voltage is too small, processing will not be possible, and if the voltage is too large, it will cause an excessive heat-affected zone, which will not only cause the edge of the hole to be uneven but also cause true decreasing of the roundness that may also make the tool consume faster, so choosing the right voltage range will have a considerable impact on the machining results.
Figure 3 is a graph showing the film thickness at different pulse frequencies under the action of a magnetic field. It can be seen from Fig. 3 that the higher the voltage frequency is, the lower the film thickness at the tip of the processing electrode is, and the film thickness at the tip of the electrode is the largest when the machining voltage is increased to 40 V, and the discharge film is 40 V. At this time, the bubble generation rate, the bubbles merge with each other to make the film thickness the largest, and as the voltage becomes larger, the film thickness becomes smaller, the bubble generation rate becomes faster due to the voltage increase, the bubble is generated in a large amount, and the electrode generates a spark discharge phenomenon. Under the action of Lorentz force and buoyancy, the bubbles will rise rapidly, and the electrolyte ion and electron exchange process were accelerated, the bubble near the electrode was quickly brought away, forming a flow field, so the film thickness is thin, and adding a magnetic field was found to improve the processing results.
2.2 Influence of magnetic field on the aperture
Since the gas flow field during electrochemical discharge machining is not stable, in this experiment, ethanol is added to the electrolyte, and the gas flow field around the electrode is changed by adding a magnetic field to investigate the related electrochemical processability. Figure 4 is a processing diagram of processing parameters at a voltage frequency of 100 Hz, a feed rate of 0.5 μm/s, a machining gap between the cathode and the workpiece of 60 μm, and a processing depth of 100 μm. Figure 4 a shows that the reaction process is more intense with the increase of voltage, the flow field around the electrode is unstable, and the processing pore size increases with the increase of voltage from 190.0 to 201.3 μm. The periphery of the aperture is jagged and uneven. Figure 4 b shows the adding of the state of the processed hole of the magnetic field; the uniform magnetic field will make the bubble flow around the electrode relatively stable, so that the circumference of the aperture is relatively flat, the voltage is found to increase, the processing aperture is obviously reduced from 186.3 to 168.8 μm, and the voltage is larger. The processing aperture is more stable, and the roundness is obviously improved under the added magnetic field.
Figure 5 is a view showing a state in which a hole is processed in the presence or absence of an added magnetic field, and a film thickness and a hole cut-off data are generated. Figure 5 shows that the magnetic field is optimally added, but the film thickness at 40 V is significantly different from the overcut. In the state of adding and not applying a magnetic field, Fig. 5a shows that the thickness of the film becomes smaller due to the voltage becoming larger. However, the cross-cutting quantity of Fig. 5b increases with the voltage value, indicating that there is still a processing difference between voltage and magnetic force. In the electrochemical discharge machining, the addition of a magnetic field for processing a film has a short growth time and a small film thickness, which is quite helpful for the micro-hole overcut and can make the flow field more stable and the roundness better. And the film thickness can be reduced because the aperture cut-off amount is reduced.
2.3 Influence of magnetic field on the taper of micro-hole
The taper of micro-hole is also one of the important processing results for electrochemical discharge machining. The ideal pore size of the inlet pore is the same as the outlet pore diameter, that is, the taper is zero, but this is not easy to achieve, because during the processing, as the processing depth is deeper, the electrolyte circulation in the micro-hole is worse, resulting in the gas bubbles being clogged between the electrodes and the micro-holes, which makes the thickness of the electrodes covered by the bubbles thicker, so that the spark discharge path becomes longer and the taper increases. Figure 6 is a side view of the machined hole at 100 Hz pulsed at different voltages for the addition of ethanol. Figure 6 a shows that in the non-magnetic state, the lower the voltage, the more obvious the taper, and the cone hole phenomenon is improved when the voltage is 48 V. Figure 6 b shows that after the magnetic force is applied, the taper phenomenon is reduced to a voltage of 44 V, and the overall taper phenomenon is also improved compared with the unmagnetized force. Figure 7 is a comparison curve after measuring the taper hole. The taper of micro-hole is the largest at 40 V. When the machining voltage is 48 V, the overall taper is the smallest. The inference should be that the large voltage provides sufficient power to make the cone hole phenomenon. Lowering, and adding magnetic force, can improve the taper of the processing hole, the overall taper improvement is about 30%, and the magnetic force and voltage should be the key to affect the Lorentz force and thus affect the hole processing effect.
3 Conclusion
In MEMS, quartz has an important position for electronic components due to its unique optical properties. It is necessary to make specific microstructures such as micro-holes and grooves on the quartz material, and the conventional processing method is liable to cause residual stress and breakage. In this paper, the electrochemical discharge machining of quartz glass is carried out by electrochemical discharge machining, and the film growth of the process is discussed. The influence of the gas film state on the processing result is very large. If the film formation can be effectively limited, the processing range and the accuracy can also be controlled. In this process, the addition of ethanol will reduce the conductivity, but the angle between the electrode and the bubble is reduced, and the thickness of the film can be reduced, so that the waveform of the square wave power source is stabilized, and the smoothing effect of the electrochemical process is improved. In addition, when the charged particles move relative to the magnetic field, the particles will be subjected to the Lorentz force, which will make the bubbles and flow generated around the electrode more stable. During the processing, it is found that the circumference of the aperture is relatively flat and the roundness is obviously improved. The taper of the machined hole is improved, and the amount of undercut of the hole is reduced. Therefore, the addition of ethanol to the KOH electrolyte and the external magnetic field on the tool can improve the related processing efficiency for electrochemical processing of quartz materials.
References
Basak I, Ghosh A (1997) Mechanism of material removal in electrochemical discharge machining: a theoretical model and experimental verification. J Mater Process Technol 71:350–359
Jain VK, Dixit PM, Pandey PM (1999) On the analysis of the electro-chemical spark machining process-test of machining on composite materials. Int J Mach Tools Manuf 39:165–186
Kulkarni A, Sharan R, Lal GK (2002) An experimental study of discharge mechanism in electrochemical discharge machining. Int J Mach Tools Manuf 42:1121–1127
Fascio V, Langen HH, Bieuler H, Comninellis C (2003) Investigations of the spark assisted chemical engraving. Electrochem Commun 5:203–207
Wüthrich R, Comninellis C, Bleuler H (2005) Bubble evolution on vertical electrodes under extreme current densities. Electrochim Acta 50:5242–5246
Wüthrich R, Hof LA (2006) The gas film in spark assisted chemical engraving (SACE) - a key element for micro-machining applications. Int J Mach Tools Manuf 46:828–835
Maillard P, Despont B, Bleuler H, Wüthrich R (2007) Geometrical characterization of micro-holes drilled in glass by gravity-feed with spark assisted chemical engraving (SACE). J Micromech Microeng 17:1343–1349
Yang CK, Cheng CP, Mai CC, Wang AC, Hung JC, Yan BH (2010) Effect of surface roughness of tool electrode materials in ECDM performance. Int J Mach Tools Manuf 50:1088–1096
Jain VK, Singh YP, Kumar P, Agrawal DC (1996) Machining piezoelectric (PZT) ceramics using an electrochemical spark machining (ECSM) process. J Mater Process Technol 58:24–31
Zheng ZP, Cheng WH, Huang FY, Yan BH (2007) 3D microstructuring of Pyrex glass using the electrochemical discharge machining process. J Micromech Microeng 17:960–966
Yang CT, Ho SS, Yan BH (2001) Micro hole machining of borosilicate glass through electrochemical discharge machining (ECDM). Key Eng Mater 196:149–166
Yang CK, Wu KL, Hung JC, Lee SM, Lin JC, Yan BH (2011) Enhancement of ECDM efficiency and accuracy by spherical tool electrode. Int J Mach Tools Manuf 51:528–535
Cheng CP, Wu L, Mai CC, Hsu YS, Yan BH (2010) Magnetic field-assisted electrochemical discharge machining. J Micromech Microeng 20:075019
Sabahi N, Hajian M, Razfar MR (2018) Experimental study on the heat-affected zone of glass substrate machined by electrochemical discharge machining (ECDM) process. Int J Adv Manuf 97:1557–1564
Hourng LW, Lin CI, Lee BG (2014) The improvement of machining accuracy on quartz and glasses by electrochemical discharge machining. Appl Mech Mater 472:682–687
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, MY., Tsai, TH., Hourng, LW. et al. The effects of magnetic field and ethanol addition on the electrochemical discharge machining. Int J Adv Manuf Technol 105, 2461–2467 (2019). https://doi.org/10.1007/s00170-019-04413-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00170-019-04413-7