Abstract
Purpose
Interscalene brachial plexus block (ISB) is one of the most commonly used regional blocks in relieving postoperative pain after arthroscopic rotator cuff repair. Dexmedetomidine (DEX) is an alpha 2 agonist that can enhance the effect of regional blocks. The aim of this study was to compare the effects of DEX combined with ISB with ISB alone on postoperative pain, satisfaction, and pain-related cytokines within the first 48 h after arthroscopic rotator cuff repair.
Methods
Fifty patients with rotator cuff tears who had undergone arthroscopic rotator cuff repair were enrolled in this single center, double-blinded randomized controlled trial study. Twenty-five patients were randomly allocated to group 1 and received ultrasound-guided ISB using a mixture of 1 ml (100 μg) of DEX and 8 ml of 0.75% ropivacaine preemptively. The other 25 patients were allocated to group 2 and underwent ultrasound-guided ISB alone using a mixture of 1 ml of normal saline and 8 ml of ropivacaine. The visual analog scale (VAS) for pain and patient satisfaction (SAT) scores were checked within 48 h postoperatively. The plasma interleukin (IL)-6, -8, -1β, cortisol, and substance P levels were also measured within 48 h, postoperatively.
Results
Group 1 showed a significantly lower mean VAS score and a significantly higher mean SAT score than group 2 at 1, 3, 6, 12, and 18 h postoperatively. Compared with group 2, group 1 showed a significantly lower mean plasma IL-6 level at 1, 6, 12, and 48 h postoperatively and a significantly lower mean IL-8 level at 1, 6, 12, 24, and 48 h postoperatively. The mean timing of rebound pain in group 1 was significantly later than that in group 2 (12.7 h > 9.4 h, p = 0.006).
Conclusions
Ultrasound-guided ISB with DEX in arthroscopic rotator cuff repair led to a significantly lower mean VAS score and a significantly higher mean SAT score within 48 h postoperatively than ISB alone. In addition, ISB with DEX showed lower mean plasma IL-6 and IL-8 levels than ISB alone within 48 h postoperatively, with delayed rebound pain.
Level of evidence
I.
Trial Registration
2013-112, ClinicalTrials.gov Identifier: NCT02766556.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Shoulder arthroscopic operations can be performed in in- or out-patient settings, but it may induce severe pain in the early postoperative period [17, 32, 33]. Therefore, it is important to control excessive postoperative pain by decreasing the pain to a tolerable level for the early return of patients to activities of daily living [35]. There are several regional blocks that can be used to relieve postoperative pain. Interscalene brachial plexus blocks (ISBs) are one of the most powerful regional blocks for shoulder operations, but they have a relatively short duration of effect [33, 51]. Suprascapular nerve blocks (SSNBs) are the most commonly used method for controlling postoperative shoulder pain, and axillary nerve blocks (ANBs) have recently been adopted as a type of regional block for shoulder pain control and are frequently used in combination with SSNB [14, 32, 49].
Dexmedetomidine (DEX), a selective agonist of α2-adrenergic receptors, can be an effective adjuvant to local anesthetics for peripheral nerve blocks [22]. Preclinical and clinical studies have described a prolonged duration of analgesia when DEX was added to bupivacaine, levobupivacaine, or ropivacaine for peripheral perineural blocks [6,7,8,9, 20, 38, 42, 55].
The alterations in pain-related cytokines after arthroscopic rotator cuff repair under ISB combined with DEX have not been studied. Therefore, the present study was designed to test the hypothesis that DEX added to ropivacaine for ISB in elective shoulder operations would enhance the duration and effect of analgesia compared with ropivacaine alone and to assess its influence on serum interleukin (IL)-6, cortisol, IL-1β, IL-8, and substance P levels.
Materials and methods
Fifty patients with a rotator cuff tear who had undergone arthroscopic rotator cuff repair between August 2014 and April 2015 were enrolled in this study. All procedures were approved by the Institutional Review Board of Chuncheon Sacred Heart Hospital and carried out in accordance with the Declaration of Helsinki (IRB number: 2013-112), and a clinical trial registration was performed. All of the patients provided written informed consent to participate in the study. Rotator cuff tears were diagnosed by preoperative magnetic resonance imaging (MRI), and the size of the rotator cuff was confirmed at the time of surgery. The indication for surgery was a symptomatic full-thickness rotator cuff tear or a > 50% thickness partial-thickness rotator cuff tear in cases of failed conservative therapy [33]. Among the patients, 25 were randomly allocated to group 1 and received ISB with ropivacaine and DEX. The remaining 25 patients were allocated to group 2 and received ISB with ropivacaine and normal saline.
The study inclusion criteria were as follows: (1) defined rotator cuff tear on preoperative MRI, which indicated repair; (2) acceptable arthroscopic surgery, including rotator cuff repair; (3) patients > 20 years; and (4) acceptable routine regional blocks and patient-controlled analgesia (PCA). The exclusion criteria involved patients who: (1) did not undergo arthroscopic rotator cuff repair; (2) stopped PCA before 48 h postoperatively due to side effects; (3) had a concomitant operation for a Bankart lesion; (4) had a history of shoulder operation or fracture; (5) had a concomitant neurological disorder around the shoulder; (6) underwent conversion to open surgery from the arthroscopy; (7) had contraindications for the routine regional blocks used in this study; or (8) had an known allergy or hypersensitivity against ropivacaine or dexmedetomidine, including other amino-amide local anesthetics or α2-adrenoceptor agonists.
Data collection and outcome measures
The visual analog scale (VAS) pain score, American Shoulder and Elbow Surgeons Shoulder Score, Constant score, height, weight, and plasma cortisol, IL-6, IL-8, IL-1β, and substance P levels were checked preoperatively, and ISB was performed preemptively under ultrasound guidance at the end of surgery. PCA was set at a fixed dose (0.05 μg/kg loading dose and 0.03 μg/min/kg continuous dose of fentanyl) to remove the effect of varying amounts of PCA [32, 33]. The VAS pain and patient satisfaction (SAT) scores were checked at 1, 3, 6, 12, 18, 24, 36, and 48 h postoperatively by an independent orthopedic resident who was blinded to whether the patient received DEX during the surgery. The VAS pain score was based on a scale from 0 to 10, where 0 indicated no pain and 10 indicated severe pain [32, 33]. The SAT score also ranged from 0 to 10, where 0 indicated unsatisfactory and 10 indicated very satisfactory [32, 33]. Plasma cortisol, IL-6, IL-8, IL-1β, and substance P levels were checked at 1, 6, 12, 24, and 48 h, postoperatively. The blood analysis was performed by laboratory staff who were blinded to the study. A 3-ml blood sample was retrieved from the peripheral vein of the contralateral upper limb or both lower limbs per sampling, and 3.8% sodium citrate (Sigma, St. Louis, MO, USA) was immediately added to the blood collection as an anticoagulant. The blood was processed and stored at 4 °C. The whole blood was centrifuged at 3000 rpm for 10 min. The plasma was separated from the blood cell layer and stored at − 40 °C. The frozen plasma had been thawed to 4 °C before the levels of blood markers were checked using several kits (Cortisol Parameter Assay Kit, Human IL-6 Quantikine ELISA Kit, Human CXCL8/IL-8 Quantikine ELISA Kit, Human IL-1 beta/IL-1F2 Quantikine ELISA Kit, and Substance P Parameter Assay Kit; R&D SYSTEMS, Minneapolis, Minnesota, USA). The precision of the five blood markers is as follows: cortisol: intra-assay 5.4–9.2, interassay 9.3–21.2; IL-6: intra-assay 1.6–4.2, interassay 3.3–6.4; IL-8: intra-assay 5.4–6.5; IL-1β: intra-assay 3.0–7.5, interassay 5.7–8.4; and substance P: intra-assay 3.5–8.4, interassay 9.4–15.1 coefficient of variation (CV; %). The primary outcome measure was VAS, and the secondary outcome measures were SAT and the levels of plasma cortisol, IL-6, IL-8, IL-1β, and substance P. Postoperative rebound pain was confirmed if there was an increase in the VAS pain score between 1 and 48 h, postoperatively [25, 33].
Interventions
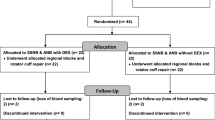
Double-blinded randomization was performed as follows. The 64 patients who met the inclusion criteria were randomly assigned to 1 of 2 groups depending on treatment. Randomization was performed with a computer random sequence generator by an independent nurse who prepared the syringe for the ISB treatment according to the group assignment. The patient and all of the medical staff who participated in the surgery were blinded to the assignments. Four patients without rotator cuff tears were excluded after the surgery. Two patients were excluded due to a history of ipsilateral operation history. One patient was excluded due to the early termination of PCA before 48 h postoperatively. One patient was excluded due to concomitant open reduction and internal fixation for ipsilateral os acromiale. One patient was excluded due to concomitant ipsilateral operation for a Bankart lesion. Four patients were excluded due to the omission of blood sampling or hemolysis of the blood sample. One patient was excluded, because she was unable to receive the ISB due to an enlarged thyroid. Thus, in total, 50 patients were included. Twenty-five patients were in group 1, and the remaining 25 patients were in group 2 (Fig. 1).
ISB was performed preemptively immediately after general anesthesia was induced by one anesthesiologist under ultrasound guidance (S-Nerve™, SonoSite, Bothell, WA, USA) once the patient was placed in the supine position. Nerves were stimulated with a commercially available nerve stimulator (Pajunk®, Geisingen, Germany). A thin layer of sterile ultrasound transmission gel (Sung Heung Corp., Pucheon, Kyungki-do, Republic of Korea) was placed between the ultrasound linear transducer and the skin immediately after preparing the skin with povidone–iodine solution [33]. The superior, middle, and inferior trunks of the brachial plexus were identified approximately 2 cm above the clavicle. A 50-mm 22-gauge needle (Uni-Plex NanoLine, Pajunk®, Geisingen, Germany) was introduced percutaneously using the out-of-plane technique. The needle was placed beside each trunk in succession, and the mixture of ropivacaine with DEX or saline (3 ml) was injected into each site. For group 1, a mixture of 8 ml of ropivacaine and 1 ml (100 μg) of DEX was used, and for group 2, a mixture of 8 ml of ropivacaine and 1 ml of normal saline was used. The needle was retracted slightly if a motor response < 0.2 mA was triggered. If contractions continued, a negative aspiration test was performed, and ropivacaine was injected slowly. Then, we turned the current back up to 1.0 mA after the injection. Ropivacaine was not injected during stimulation at an intensity of < 0.2 mA to avoid an intraneural injection [33, 50].
Operative treatment
All of the patients underwent arthroscopic rotator cuff repair and subacromial decompression. All of the procedures were performed by one surgeon. Four routine arthroscopic portals (anterior, posterior, lateral, and posterolateral) were used during the surgery. Arthroscopic subacromial decompression was performed with acromioplasty, followed by the removal of spurs after the bursectomy in all patients. The suture bridge technique was used for rotator cuff repair with 5.0-mm Bio-Corkscrew suture anchors (Arthrex, Naples, FL, USA) and a 4.75-mm Bio-SwiveLock device (Arthrex). Tendon-to-tendon sutures were occasionally used for small to medium tears located around the musculotendinous junction [32, 45]. Tenotomy or tenodesis of the long head of the biceps, distal clavicle resection, and anterior capsulectomy were performed simultaneously based on concomitant diseases. The indication of distal clavicle resection is symptomatic acromioclavicular arthritis, and the indication for anterior capsulectomy is adhesive capsulitis. Adhesive capsulitis was defined as passive forward elevation < 100° and passive external rotation at the side < 30° [32, 33, 43]. The operation time was the duration between the first skin incision and suture of the operative wound.
Postoperative rehabilitation
A shoulder-immobilizing sling with an abduction pillow was prescribed to each patient postoperatively, with instructions to maintain the shoulder at 30°–40° of internal rotation and 20° abduction. Postoperative rehabilitation was individualized according to the size of the tear and the tissue quality of the torn rotator cuff. All of the patients were allowed passive forward elevation using a pulley 48 h postoperatively, immediately after PCA had been removed [32, 33, 44].
Statistical analysis
Fifty patients (25 patients in each group) would provide a statistical power of 80% with a two-sided α level of 0.05 to detect a significant difference in VAS at 12 h postoperatively, assuming an effect size of 0.84 [mean difference, 1.85; standard deviation (SD), 2.21]. This calculation was based on the mean and SD of VAS observed in a pilot study of 20 patients taken at 12 h postoperatively. The normally distributed data between the groups were analyzed using independent sample t test. Otherwise, the nonparametric Mann–Whitney U test was used. Probable factors that may have affected rebound phenomenon characteristics were analyzed by univariate logistic regression. The statistical analysis was performed using IBM SPSS Statistics 22 (IBM Corp., Armonk, NY, USA). p < 0.05 was considered statistically significant.
Results
The demographic data, such as the mean age, body mass index (BMI), and symptom duration, were similar between the two groups (Table 1). In addition, there were no significant differences in the operative data between the two groups (Table 2). Although single row repair and side-to-side tendon repair were performed 3 times in group 1 only, there was no statistically significant difference between the two groups.
Group 1 showed a significantly lower mean VAS and a significantly higher mean SAT than group 2 at 1, 3, 6, 12, and 18 h postoperatively (VAS: 1.2 < 2.1, 1.2 < 2.4, 1.6 < 4.5, 4.2 < 6.1, and 4.5 < 5.5 (p = 0.017, p = 0.015, p < 0.001, p = 0.003 and p = 0.040); SAT: 8.3 > 7.6, 8.4 > 7.6, 7.8 > 5.6, 6.2 > 4.6, and 6.2 > 5.4 (p = 0.034, p = 0.032, p < 0.001, p = 0.001, and p = 0.049), respectively) (Table 3). Compared with group 2, group 1 showed a significantly lower mean plasma IL-6 level at 1, 6, 12, and 48 h postoperatively (7 < 10, 15 < 25, 20 < 33, and 13 < 19) and a significantly lower mean IL-8 level at 1, 6, 12, 24, and 48 h postoperatively (12 < 13.0, 11 < 13, 11 < 14, 11 < 13, and 12 < 15). Moreover, compared with group 2, group 1 showed a significantly lower mean plasma cortisol level at 6 h postoperatively (116.9 < 143.0) and a significantly lower plasma substance P level at 1 h postoperatively (112 < 128) (Table 4). All of the patients in group 1 and group 2 showed rebound pain one time. The mean timing of rebound pain in group 1 was significantly later than that in group 2 (12.7 h > 9.4 h, p = 0.006), and the mean level of rebound pain of the two groups was not significantly different (4.1 < 4.8, n.s.) (Table 5). The mean timing of rebound pain was correlated with the combined DEX administration in the univariate logistic regression (p = 0.010, odds ratio = 1.223). However, the mean level of rebound pain did not show a significant correlation with the combined DEX administration (n.s., odds ratio = 0.753). All of the rebound pain occurred between 3 and 36 h postoperatively.
Discussion
The most important finding of the present study was that the combination of ultrasound-guided ISB with DEX tended to decrease the postoperative VAS and SAT scores after arthroscopic shoulder surgery compared to those of ISB alone within the first 48 h postoperatively. Pain is a major factor influencing the duration of hospital stays, and postoperative pain control is important for early rehabilitation and the early return of patients to activities of daily living [32, 33]. Several methods can be used for controlling postoperative pain after shoulder surgery. PCA can decrease some of the postoperative pain, but it may induce several side effects, such as nausea, vomiting, and somnolence [57]. A continuous analgesic infusion pump is an effective method for relieving postoperative shoulder pain. However, leakage, occasional equipment malfunction, and infection can happen [5, 33, 39]. Several regional blocks, including ISB, SSNB, and ANB, can be used [14, 23, 25, 32,33,34,35]. ISB is an efficacious method, but may lead to pneumothorax or phrenic nerve palsy and has a shorter duration of effect than SSNB [31, 33, 35, 56]. ISB can be performed using a single injection or continuous injections with a catheter [23, 31, 33, 51]. A randomized controlled trial showed that compared with single-injection ISB, continuous ISB leads to significantly shorter hospital stays; however, the catheter may dislocate during continuous ISB, and patients frequently feel uncomfortable [37, 51].
In this study, the combination of ultrasound-guided ISB with DEX tended to decrease postoperative VAS scores and increase postoperative SAT scores after arthroscopic shoulder surgery compared to those of ISB alone within the first 48 h postoperatively. DEX added to ropivacaine for ISB increased the duration of the nerve block. The mechanism by which α2-adrenergic receptor agonists produce analgesia and sedation is not fully understood but is likely multifactorial. Peripherally, α2 agonists produce analgesia by reducing the release of norepinephrine and causing α2 receptor-independent inhibitory effects on nerve fiber action potentials. Centrally, α2 agonists produce analgesia and sedation by inhibiting the release of substance P in the nociceptive pathway at the level of the dorsal root neuron and by activating α2 adrenoceptors in the locus coeruleus [1, 10, 22, 38].
Pain-related blood markers include proinflammatory cytokines, anti-inflammatory cytokines, cortisol, substance P, etc. [3, 4, 18, 21, 26, 28, 36, 47]. Cytokines influence the activity, differentiation, proliferation, and survival of immune cells, as well as regulate the production and activity of other cytokines that can increase (proinflammatory) or decrease (anti-inflammatory) the inflammatory response. Proinflammatory cytokines include IL-1, -2, -6, -7, and -8, and tumor necrosis factor (TNF), and anti-inflammatory cytokines include IL-4, -10, and -13, and transforming growth factor (TGF)-β [13, 14, 16, 18, 28, 30, 40, 52]. It has also been reported that salivary and plasma cortisol levels are closely related to stress or pain [16, 26]. Substance P has been thought to be related to pain and stem cell recruitment [27, 36]. Pain and the immune system influence each other, making it difficult to determine whether blocking nociception contributes to a reduction in the production of proinflammatory cytokines or vice versa, with a reduction in the formation of proinflammatory cytokines resulting in less severe pain [53].
The mean physiological blood levels of the blood markers are as follows: cortisol: 12.6 µg/ml; IL-6: 0.5–5.1 pg/ml; IL-8: 7.526–29.3 pg/ml; IL-1β: 0.12–16 pg/ml; and substance P: 18 pg/ml [2, 11, 12, 19, 24, 29, 41, 48]. The mean physiological blood levels are lower than those in the present study, because the operation caused pain. The mean plasma IL-6 level in patients without any nerve block after arthroscopic rotator cuff repair was 24.3 pg/ml [54]. This value is located between those of groups 1 and 2 in the present study. The mean plasma IL-6 and IL-1β levels in patients who experienced only narcosis were 2.04 and 38.53 pg/ml, respectively [58]. According to these values, the mean blood level of IL-6 was lower and the mean level of IL-1β was higher than those of the two groups in the present study.
Rebound pain has been reported in several studies in which regional blocks, such as ISB, SSNB, and ANB, were used to control pain after arthroscopic shoulder surgery [17, 25, 32, 33, 35]. One study showed that ANB combined with SSNB causes fewer rebound phenomena than that of SSNB alone [32]. Another study suggested that the combination of ISB and SSNB tended to delay the mean time of rebound pain and subsequently reduced mean rebound pain severity compared to that of ISB alone [23]. In the present study, the mean time of rebound pain occurred later with the combination of ultrasound-guided ISB and DEX than that with the ultrasound-guided ISB alone, but the mean level of rebound pain was not significantly different between the two groups.
This study had some limitations. First, the VAS and SAT scores were subjective. However, all previous studies on preemptive regional blocks for postoperative pain control used these subjective scoring systems. Second, this study does not include a control group that underwent rotator cuff repair without an interscalene block at all. However, if we included a control group that underwent arthroscopic rotator cuff repair without ISB, this control group would not receive any benefits for postoperative pain relief using the regional block. To avoid the moral problem and facilitate the recruitment of participants, the two groups were determined to be ISB only and ISB with DEX.
There are several merits in the present study. First, no previous studies have been performed on the effect of DEX combined with ultrasound-guided ISB for relieving postoperative pain after arthroscopic rotator repair compared to ISB alone using pain-related blood markers. Therefore, adequate blood markers can be selected for evaluating pain and may be utilized for grading the pain objectively. The selected blood markers for pain can be used as a parameter to determine the amount of narcosis. Using blood markers, the problem of narcotic addiction can be reduced [46]. Second, the present study was a double-blinded randomized controlled trial in which a power analysis was performed. Third, one anesthesiologist conducted all of the ultrasound-guided ISBs using nerve stimulation and one orthopedic surgeon who specialized in shoulder arthroscopic surgery performed all the surgeries. Finally, PCA was set to a low fixed dose to remove the effect of differing quantities of the analgesic. DEX combined with ISB showed a synergistic effect in relieving postoperative pain in arthroscopic rotator cuff repair. Based on the results of the present study, evidence on objective blood pain markers might be obtained.
Conclusions
Ultrasound-guided ISB with DEX in arthroscopic rotator cuff repair showed a significantly lower mean VAS score and a significantly higher mean SAT score within 48 h postoperatively than ISB alone. In additional ISB with DEX showed lower mean plasma IL-6 and IL-8 levels than ISB alone within 48 h postoperatively, with delayed rebound pain.
References
Abdallah FW, Brull R (2013) Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: a systematic review and meta-analysis. Br J Anaesth 110(6):915–925
Bamba T, Yoshioka U, Inoue H, Iwasaki Y, Hosoda S (1994) Serum levels of interleukin-1 beta and interleukin-6 in patients with chronic pancreatitis. J Gastroenterol 29(3):314–319
Beilin B, Bessler H, Mayburd E et al (2003) Effects of preemptive analgesia on pain and cytokine production in the postoperative period. Anesthesiology 98(1):151–155
Brenn D, Richter F, Schaible HG (2007) Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: an inflammatory mechanism of joint pain. Arthritis Rheum 56(1):351–359
Brown SL, Morrison AE (2004) Local anesthetic infusion pump systems adverse events reported to the Food and Drug Administration. Anesthesiology 100(5):1305–1307
Brummett CM, Amodeo FS, Janda AM, Padda AK, Lydic R (2010) Perineural dexmedetomidine provides an increased duration of analgesia to a thermal stimulus when compared with a systemic control in a rat sciatic nerve block. Reg Anesth Pain Med 35(5):427–431
Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R (2011) Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology 115(4):836–843
Brummett CM, Norat MA, Palmisano JM, Lydic R (2008) Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology 109(3):502–511
Brummett CM, Padda AK, Amodeo FS, Welch KB, Lydic R (2009) Perineural dexmedetomidine added to ropivacaine causes a dose-dependent increase in the duration of thermal antinociception in sciatic nerve block in rat. Anesthesiology 111(5):1111–1119
Brummett CM, Williams BA (2011) Additives to local anesthetics for peripheral nerve blockade. Int Anesthesiol Clin 49(4):104–116
Campbell DE, Bruckner P, Tustin NB, Tustin R 3rd, Douglas SD (2009) Novel method for determination of substance P levels in unextracted human plasma by using acidification. Clin Vaccine Immunol. 16(4):594–596
Campbell DE, Raftery N, Tustin R 3rd, etc. (2006) Measurement of plasma-derived substance P: biological, methodological, and statistical considerations. Clin Vaccine Immunol 13(11):1197–1203
Cardozo LB, Cotes LC, Kahvegian MA et al (2014) Evaluation of the effects of methadone and tramadol on postoperative analgesia and serum interleukin-6 in dogs undergoing orthopaedic surgery. BMC Vet Res 10:194
Checcucci G, Allegra A, Bigazzi P, Gianesello L, Ceruso M, Gritti G (2008) A new technique for regional anesthesia for arthroscopic shoulder surgery based on a suprascapular nerve block and an axillary nerve block: an evaluation of the first results. Arthroscopy 24(6):689–696
Chen YW, Tzeng JI, Lin MF, Hung CH, Hsieh PL, Wang JJ (2014) High-frequency transcutaneous electrical nerve stimulation attenuates postsurgical pain and inhibits excess substance P in rat dorsal root ganglion. Reg Anesth Pain Med 39(4):322–328
Choi JC, Lee JH, Choi E, Chung MI, Seo SM, Lim HK (2014) Effects of seasonal differences in testosterone and cortisol levels on pain responses under resting and anxiety conditions. Yonsei Med J 55(1):216–223
DeMarco JR, Componovo R, Barfield WR, Liles L, Nietert P (2011) Efficacy of augmenting a subacromial continuous-infusion pump with a preoperative interscalene block in outpatient arthroscopic shoulder surgery: a prospective, randomized, blinded, and placebo-controlled study. Arthroscopy 27(5):603–610
de Oliveira CM, Sakata RK, Issy AM, Gerola LR, Salomao R (2011) Cytokines and pain. Rev Bras Anestesiol 61(2):255–9, 60–65, 137–142
Du S, Sun Y, Zhao B (2018) Interleukin-6 serum levels are elevated in individuals with degenerative cervical myelopathy and are correlated with symptom severity. Med Sci Monit 24:7405–7413
Esmaoglu A, Yegenoglu F, Akin A, Turk CY (2010) Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg 111(6):1548–1551
Frank LA, Kunkle GA, Beale KM (1992) Comparison of serum cortisol concentration before and after intradermal testing in sedated and nonsedated dogs. J Am Vet Med Assoc 200(4):507–510
Fritsch G, Danninger T, Allerberger K et al (2014) Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med 39(1):37–47
Gautier P, Vandepitte C, Ramquet C, DeCoopman M, Xu D, Hadzic A (2011) The minimum effective anesthetic volume of 0.75% ropivacaine in ultrasound-guided interscalene brachial plexus block. Anesth Analg 113(4):951–955
Guo GH, Dong J, Yuan XH, Dong ZN, Tian YP (2013) Clinical evaluation of the levels of 12 cytokines in serum/plasma under various storage conditions using evidence biochip arrays. Mol Med Rep 7(3):775–780
Jeske HC, Krallinger F, Wambacher M et al (2011) A randomized study of the effectiveness of suprascapular nerve block in patient satisfaction and outcome after arthroscopic subacromial decompression. Arthroscopy 27(10):1323–1328
Karbic VO, Skoda M, Antoncic D, Kristofic I, Komar D, Trobonjaca Z (2014) Gabapentin-induced changes of plasma cortisol level and immune status in hysterectomized women. Int Immunopharmacol 23(2):530–536
Kim JH, Jung Y, Kim BS, Kim SH (2013) Stem cell recruitment and angiogenesis of neuropeptide substance P coupled with self-assembling peptide nanofiber in a mouse hind limb ischemia model. Biomaterials 34(6):1657–1668
Kim SJ, Kim JE, Kim SH et al (2016) Therapeutic effects of neuropeptide substance P coupled with self-assembled peptide nanofibers on the progression of osteoarthritis in a rat model. Biomaterials 74:119–130
Kleiner G, Marcuzzi A, Zanin V, Monasta L, Zauli G (2013) Cytokine levels in the serum of healthy subjects. Mediat Inflamm 2013:434010
Kosek E, Altawil R, Kadetoff D et al (2015) Evidence of different mediators of central inflammation in dysfunctional and inflammatory pain–interleukin-8 in fibromyalgia and interleukin-1 beta in rheumatoid arthritis. J Neuroimmunol 280:49–55
Lee JH, Cho SH, Kim SH et al (2011) Ropivacaine for ultrasound-guided interscalene block: 5 mL provides similar analgesia but less phrenic nerve paralysis than 10 mL. Can J Anaesth 58(11):1001–1006
Lee JJ, Kim DY, Hwang JT et al (2014) Effect of ultrasonographically guided axillary nerve block combined with suprascapular nerve block in arthroscopic rotator cuff repair: a randomized controlled trial. Arthroscopy 30(8):906–914
Lee JJ, Hwang JH, Kim DY et al (2017) Effects of arthroscopy-guided suprascapular nerve block combined with ultrasound-guided interscalene brachial plexus block for arthroscopic rotator cuff repair: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 25(7):2121–2128
Lee JJ, Yoo YS, Hwang JT et al (2015) Efficacy of direct arthroscopy-guided suprascapular nerve block after arthroscopic rotator cuff repair: a prospective randomized study. Knee Surg Sports Traumatol Arthrosc 23(2):562–566
Lee SM, Park SE, Nam YS et al (2012) Analgesic effectiveness of nerve block in shoulder arthroscopy: comparison between interscalene, suprascapular and axillary nerve blocks. Knee Surg Sports Traumatol Arthrosc 20(12):2573–2578
Lisowska B, Siewruk K, Lisowski A (2016) Substance P and acute pain in patients undergoing orthopedic surgery. PLoS O ne 11(1):e0146400
Marhofer D, Marhofer P, Triffterer L, Leonhardt M, Weber M, Zeitlinger M (2013) Dislocation rates of perineural catheters: a volunteer study. Br J Anaesth 111(5):800–806
Marhofer D, Kettner SC, Marhofer P, Pils S, Weber M, Zeitlinger M (2013) Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: a volunteer study. Br J Anaesth 110(3):438–442
Matsen FA 3rd, Papadonikolakis A (2013) Published evidence demonstrating the causation of glenohumeral chondrolysis by postoperative infusion of local anesthetic via a pain pump. J Bone Jt Surg Am 95(12):1126–1134
Mendieta D et al (2016) IL-8 and IL-6 primarily mediate the inflammatory response in fibromyalgia patients. J Neuroimmunol 290:22–25
Natung T, Keditsu A (2015) Comparison of serum cortisol and testosterone levels in acute and chronic central serous chorioretinopathy. Korean J Ophthalmol 29(6):382–388
Obayah GM, Refaie A, Aboushanab O, Ibraheem N, Abdelazees M (2010) Addition of dexmedetomidine to bupivacaine for greater palatine nerve block prolongs postoperative analgesia after cleft palate repair. Eur J Anaesthesiol 27(3):280–284
Oh JH, Oh CH, Choi JA, Kim SH, Kim JH, Yoon JP (2011) Comparison of glenohumeral and subacromial steroid injection in primary frozen shoulder: a prospective, randomized short-term comparison study. J Shoulder Elbow Surg 20(7):1034–1040
Park JY, Lhee SH, Oh KS, Kim NR, Hwang JT (2012) Is arthroscopic coracoplasty necessary in subcoracoid impingement syndrome? Arthroscopy 28(12):1766–1775
Park JY, Lhee SH, Oh KS, Moon SG, Hwang JT (2013) Clinical and ultrasonographic outcomes of arthroscopic suture bridge repair for massive rotator cuff tear. Arthroscopy 29(2):280–289
Preuss CV, Kalava A, King KC (2019) Prescription of controlled substances: benefits and risks. StatPearls Publishing, Treasure Island
Radzikowska E, Roży A, Jaguś P et al (2016) Cryptogenic organizing pneumonia: IL-1beta, IL-6, IL-8, and TGF- beta1 serum concentrations and response to clarithromycin treatment. Adv Exp Med Biol 911:77–85
Robak T, Gladalska A, Stepień H, Robak E (1998) Serum levels of interleukin-6 type cytokines and soluble interleukin-6 receptor in patients with rheumatoid arthritis. Mediat Inflamm 7(5):347–353
Rothe C, Lund J, Jenstrup MT, Lundstrom LH, Lange KH (2012) Ultrasound-guided block of the axillary nerve: a case series of potential clinical applications. Acta Anaesthesiol Scand 56(7):926–930
Salem MH, Winckelmann J, Geiger P, Mehrkens HH, Salem KH (2012) Electrostimulation with or without ultrasound-guidance in interscalene brachial plexus block for shoulder surgery. J Anesth 26(4):610–613
Salviz EA, Xu D, Frulla A et al (2013) Continuous interscalene block in patients having outpatient rotator cuff repair surgery: a prospective randomized trial. Anesth Analg 117(6):1485–1492
Schaible HG, von Banchet GS, Boettger MK et al (2010) The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann N Y Acad Sci 1193:60–69
Shavit Y, Fridel K, Beilin B (2006) Postoperative pain management and proinflammatory cytokines: animal and human studies. J Neuroimmune Pharmacol 1(4):443–451
Shinoda T, Shibata Y, Izaki T, Shitama T, Naito M (2009) A comparative study of surgical invasion in arthroscopic and open rotator cuff repair. J Shoulder Elbow Surg 18(4):596–599
Swami SS, Keniya VM, Ladi SD, Rao R (2012) Comparison of dexmedetomidine and clonidine (alpha2 agonist drugs) as an adjuvant to local anaesthesia in supraclavicular brachial plexus block: a randomised double-blind prospective study. Indian J Anaesth 56(3):243–249
Thackeray EM et al (2013) Diaphragm function after interscalene brachial plexus block: a double-blind, randomized comparison of 0.25% and 0.125% bupivacaine. J Shoulder Elbow Surg 22(3):381–386
Watcha MF, White PF (1992) Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology 77(1):162–184
Whitaker EE, Christofi FL, Quinn KM, etc. (2017) Selective induction of IL-1β after a brief isoflurane anesthetic in children undergoing MRI examination. J Anesth 31(2):219–224
Acknowledgements
The authors thank Jun Sub Jung, a research agent for analyzing the blood samples. This work was supported by the Hallym University Research Fund (HURF-2015-35; J. J. L. and J. S. J.).
Funding
This work was supported by Hallym University Research Fund (HURF-2015-35; J. J. L. and J. S. J.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of Chuncheon Sacred Heart Hospital (IRB number: 2013-112) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hwang, JT., Jang, J.S., Lee, J.J. et al. Dexmedetomidine combined with interscalene brachial plexus block has a synergistic effect on relieving postoperative pain after arthroscopic rotator cuff repair. Knee Surg Sports Traumatol Arthrosc 28, 2343–2353 (2020). https://doi.org/10.1007/s00167-019-05799-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-019-05799-3