Abstract
Purpose
To compare the effectiveness and safety of intra-articular injections of autologous expanded mesenchymal stromal stem cells alone (MSCs), or in combination with platelet-rich plasma (MSCs + PRP), in patients with knee osteoarthritis.
Methods
Eighteen patients (57.6 ± 9.6 years) with radiographic symptomatic knee osteoarthritis (Dejour grades II–IV) were randomized to receive intra-articular injections of MSCs (n = 9) or MSCs + PRP (n = 9). Injections were performed 2–3 weeks after bone marrow aspiration (± 80–100 ml) which was obtained from both posterior iliac crests.
Results
The Knee Injury and Osteoarthritis Outcome Score (KOOS) improved significantly throughout the 12 months for both groups (p < 0.05). No statistically significant differences between groups were found in KOOS subscales and global score improvements at 12-month end-point (n.s.). The MSCs group showed significant improvements in the pain, function and daily living activities, and sports and recreational activities subscales (p < 0.05). Similarly, the MSCs + PRP group showed significant improvements in the pain, function and daily living activities and quality of life subscales (p < 0.05). The average number of fibroblast colony forming units (CFU-F) was 56.8 + 21.9 for MSCs group and 50.7 ± 21.7 for MSCs + PRP group. Minimal adverse effects were seen in both groups (10 adverse events, in 5 patients).
Conclusions
Intra-articular injections of expanded MSCs alone or in combination with PRP are safe and have a beneficial effect on symptoms in patients with symptomatic knee osteoarthritis. Adding PRP to the MSCs injections did not provide additional benefit. These results are encouraging and support the recommendation of this minimally invasive procedure in patients with knee osteoarthritis, without requiring hospitalization. The CFU-F results may be used as reference for future research.
Level of evidence
Prospective cohort study, Level II.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knee osteoarthritis (OA) is a chronic progressive disease affecting more than 20% of people older than 45 years [24], involving progressive degeneration of the articular cartilage and subchondral bone, often accompanied by synovitis [18]. A myriad of different treatments have been proposed in the scientific literature [15, 44], and current treatment options include conservative and surgical procedures in which the main objective is to relieve pain and improve function; however, none of these treatment options affect the natural progression of the disease [13]. Nonsurgical options display short-term efficacy and, in severe joint degenerative disease, total knee replacement has become the standard treatment [3, 25]. Due to the avascular nature of articular cartilage and the limited self-renewal capacity of chondrocytes [17, 26], treatment options are generally just palliative. Reconstructive surgical options are invasive, with the potential for complications and high costs [2, 16]. The lack of a gold-standard treatment with long-term relief of symptoms, improvement of function and, at the same time, a less invasive procedure with minimum risks and relatively low costs, points out the need for novel treatment options.
More recently, the use of human bone marrow-derived mesenchymal stem cells (MSC) [5, 6], also known as stromal stem cells [31] have emerged as a potential treatment for knee OA [30, 40]. These cells are characterized by their distinct ability to adhere and grow on a substrate in vitro and to differentiate into osteoblasts, chondrocytes, adipocytes, and hematopoiesis-supporting reticular stroma when cultured under appropriate differentiation conditions [8]. Considering the degenerative and inflammatory pathophysiology of knee OA [12, 34], the stimulation of local tissue regeneration and the inhibition of immunological responses and inflammatory mediator expression by MSC therapy may be effective in treating knee OA [32]. Moreover, their ease of harvest [7] and reported safety [12, 33] make them an attractive alternative to more invasive approaches. Although early promising results have been reported after MSC treatment for knee OA [19, 22, 32, 42], there is a lack of high-level evidence regarding its effectiveness in humans [32].
Platelet-rich plasma (PRP) action on articular cartilage regeneration has been widely studied, representing a potential biological treatment for knee OA [4, 27]. PRP derives from autologous blood in which platelets are concentrated in plasma and, upon activation, it releases high concentrations of a wide range of growth factors which play key roles in tissue repair [1, 10, 11]. Particularly, transforming growth factor-beta (TGF-β), fibroblast growth factor (FGF), and insulin-like growth factor (IGF) are examples of PRP-derived growth factors that have important chondrogenic properties [39]. Prior studies have reported clinical improvements after intra-articular PRP injections for degenerative cartilage lesions and osteoarthritis [4, 20, 27]. In vitro, the use of PRP has also shown to enhance or at least preserve MSC chondrogenic capacity [29, 35]. In animal models, promising results have been reported using PRP in combination with MSCs [28, 43]. However, no clinical studies have compared treatment with MSC alone versus MSC in combination with PRP. Hence, this study aims to compare the effectiveness and safety of intra-articular injections of expanded MSC alone, or in combination with PRP, in patients with knee OA at 12-month follow-up. The effects of the combination of expanded MSCs and PRP for the treatment of symptomatic knee OA are still not known and may represent a potential useful biologic treatment for improving symptoms and function of OA patients, while potentially delaying the surgical treatment.
Materials and methods
Study design and patients
Patients over age 35 with knee OA (based on American College of Rheumatology criteria) and confirmatory radiographs (Dejour grade 1–4) [9] were included in this study. Exclusion criteria included history of diabetes mellitus, glaucoma, immunodeficiency, chronic use of oral corticosteroid or immunosuppressive therapies, history or presence of malignant disorders and/or use of chemotherapy, infection or active wound in the knee area, history of severe trauma to the knee (post-traumatic OA), presence of systemic inflammation, body mass index (BMI) greater than 40, pregnancy and any other comorbidity that prevented the surgical procedure.
Participants were included after an initial screening visit (visit #1). This included history taking, physical examination, laboratory testing, electrocardiogram, chest radiography, knee radiography [standing anterior–posterior (AP) and lateral views], knee magnetic resonance imaging (MRI) and a survey of recently used medications and supplements. All patients were also invited to complete the Portuguese version of Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire [14]. Additionally, patients were evaluated by a cardiologist for surgical risk stratification. Following this evaluation, bone marrow aspiration procedures were scheduled.
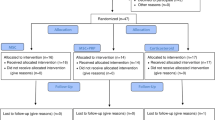
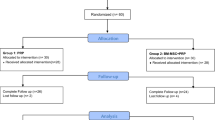
Using the Excel software (Microsoft), the included patients were randomly divided into two groups in a 1:1 ratio using central and permuted-blocks randomization (Fig. 1).
-
1.
Autologous transplantation of expanded bone marrow stromal mesenchymal cells (G1 group);
-
2.
Autologous transplantation of expanded stromal bone marrow mesenchymal cells combined with the injection of platelet-rich plasma (G2 group).
Information about the allocated group was obtained by randomization after the bone marrow aspirate and before the scheduled intra-articular injection procedure. The biologist who prepared the material to be injected had password-restricted access to the randomization schedule, which specified the allocated treatment group. The treating orthopedic surgeon, patients, and staff directly involved in the study were blinded to group assignment (G1 and G2). Only the biologist involved with the preparation of the material to be injected knew the experimental group to which the patient was allocated. During treatment preparation, the therapeutic solutions were packed in a standard syringe and covered with a protector to prevent identification of the different treatments.
Bone marrow aspirate surgical harvest procedure
Bone marrow aspirate was obtained after spinal nerve block and sedation were performed. The patient was placed in prone position and the iliac crests were prepared in aseptic conditions. Bone marrow aspirate was obtained percutaneously from both posterior iliac crests with a Jamshid needle (11G × 10 cm). The needle was introduced about 6 cm deep towards the spongy bone of the iliac crest between the inner and outer cortical tables. Approximately 80–100 ml of bone marrow were aspirated through 10-ml EDTA syringes (10 syringes used on the left posterior iliac crest side and 10 syringes used on the right side) that had been rinsed with anticoagulant (heparin). Each syringe was filled to a maximum volume of 5 ml to reduce dilution by the peripheral blood. All bone perforations were performed through the same percutaneous skin incision and were spaced about 2 cm apart to avoid dilution by aspiration of the previous region. The syringes containing bone marrow aspiration were moved to a transfer bag, where they were maintained in sterile conditions and placed in a temperature-controlled box with maintenance of temperature from 4 to 10 °C.
MSCs and PRP preparation procedures
The laboratory procedures for MSCs expansion and PRP preparation are fully described in Supplement 1.
Intra-articular injection
Intra-articular injection of MSCs (G1) or MSCs enriched with PRP (G2) was performed between 2 and 3 weeks after the bone marrow aspiration procedure. After asepsis and adequate antisepsis procedures, intra-articular injection with a 20G needle was performed in the supero-lateral region of the patella, with the patient in the supine position and the knee in extension.
Post-operative and post-injection care
After the bone marrow aspiration procedure, no functional restrictions nor special care was recommended or required. After intra-articular injection, all patients were instructed to walk with two Canadian-type crutches on the involved limb for a period of 2 weeks. Immediately, patients were instructed to initiate daily physical therapy treatment, following the same protocol for both study groups. For the first 2 weeks, the patients were non-weight bearing, but knee range of motion was not limited. Additionally, isometric quadriceps strengthening and stretching of the thigh, hip and leg muscles for deep venous thrombosis prophylaxis were performed. After 2 weeks, all activities including sports were allowed. Patients were generally recommended to continue their mild-to-moderate level of activities and to increase their activity level gradually as tolerated.
Outcome measures
Medical visits occurred before the bone marrow procedure (screening visit) and at 1, 2, 3, 6, 9 and 12 months after the experimental treatment by the orthopedic team. Pain and knee joint function were evaluated according to KOOS. Using the KOOS subscales and global score, the improvement expected (IE = 100 − initial score), the improvement observed (IO = final score − initial score) and the percentage achieved of the improvement expected (CIE = IO/IE) from baseline to 12-month follow-up was calculated for each patient. The means of these measures were calculated.
During the 12 months of follow-up, other therapies such as anti-inflammatory medications (steroid or non-steroid), hydrotherapy, heat and ultrasound or acupuncture were prohibited, to avoid influencing the results. The use of dipyrone 1 g every 6 h (analgesic non-anti-inflammatory) was allowed in case of severe pain.
Ethical approval
The research was approved by the Comissão Nacional de Ética em Pesquisa (Brazilian National Ethics in Research Committee)—CONEP, under number 14878813.4.0000.5533 and was conducted in accordance with the principles of the Declaration of Helsinki. All the participants signed the written consent form before inclusion in the study.
Statistical analysis
The statistical analysis was performed using the Statistical Package for Social Science version 22® (IBM SPSS, Inc., Chicago). The level of significance for all hypothesis tests (p) was set at 0.05. Absolute (n) and relative (%) frequencies were computed for the categorical variables. Quantitative variables with normal distribution were described using mean and standard deviation, and 95% confidence intervals (CI) for the means were estimated. The association between baseline categorical variables was determined using Fisher’s exact tests. The normality of the distribution of quantitative variables was evaluated by Kolmogorov–Smirnov’s test and Shapiro–Wilk’s test, but due the small sample size the non-parametric tests were performed in all evaluations. Differences between two independent groups for continuous variables were tested using Mann–Whitney’s test. To assess the KOOS improvement from baseline to 12-month follow-up, the Wilcoxon’s signed-rank test compared two related measures.
Results
Patient baseline characteristics
Eighteen patients (9 males and 9 females; 57.6 ± 9.6 years old) with radiographic symptomatic knee OA met the inclusion criteria and were enrolled to receive intra-articular injections of either MSCs (G1; n = 9) or MSCs + PRP (G2; n = 9). Baseline sociodemographic and clinical characteristics of both groups are summarized on Table 1. At baseline, the sample was homogeneous regarding gender, age, ROM and KOOS subscales and global scores (n.s.).
Clinical outcomes
The KOOS score improved significantly throughout the 12 months for both MSCs and MSCs + PRP groups (p < 0.05). Additionally, the KOOS score improved significantly from baseline to the first month for MSCs and MSCs + PRP groups (p < 0.05), and from the second to third month in the MSCs + PRP group (p = 0.012). The progression of mean values of the KOOS score is displayed in Fig. 2. Moreover, there was no statistically significant difference between the groups in the improvements on the KOOS subscales and global score at 12-month end-point (n.s.).
The mean percentage improvement was greater than 21% for all KOOS subscales and the global score (Table 2). The MSCs group showed significant improvements in the pain (p = 0.035), function, and daily living activities (p = 0.035) and sports and recreational activities subscales (p = 0.027). Similarly, the MSCs + PRP group showed significant improvements in the pain (p = 0.012), function and daily living activities (p = 0.017) and quality of life subscales (p = 0.027). Moreover, the global KOOS score improved 22.65 points (29%) for MSCs group (p = 0.025) and 26.4 points (30.7%) for MSCs + PRP group (p = 0.012). At final follow-up, there were no significant differences between the groups regarding the KOOS total score and respective subscales (n.s.).
Flow cytometry and fibroblast colony forming units (CFU-F)
The average number of CFU-F was 56.8 + 21.9 (range 18.3–85.0) for MSCs group and 50.7 ± 21.7 (range 19.6–85.7). Mesenchymal progenitors presented a typical myofibroblast pattern of growth. At the edge of the colonies, cells presented a stellate morphology, and at the core of the colonies, cells presented a fusiform monolayer distribution. Cells were evaluated by FACS for the expression of antigenic markers commonly associated with MSCs. After in vitro expansion, cells from all patients expressed similar levels of CD73 (> 95%), CD90 (> 95%), and CD146 (> 55%), and were negative for CD14, CD31, CD34, and CD45.
Safety and adverse events
A total of 10 adverse events (AEs) occurred in 3 patients in the MSCs group (33%) and in 2 of the MSCs + PRP group (22%). All the adverse events reported were treatment-related and none required a specific procedure or hospitalization, and were resolved without any sequelae. The MSCs group reported 5 mild AEs (joint effusion, mild knee pain, low back pain and moderate knee pain), 1 moderate AE (moderate knee pain, treated with concomitant dipyrone therapy) and 1 severe AE (intense knee pain). In the MSCs + PRP group 2 mild AEs were reported (mild knee pain) and 1 severe AE (intense knee pain, treated with concomitant dipyrone therapy). The frequency distribution of AEs reported is displayed in Table 3.
Discussion
The main finding of this double-blind randomized clinical trial is that intra-articular injections of expanded MSCs alone or in combination with PRP were safe and result in significant clinical improvement in patients with knee OA, up to 12 months. However, there were no statistically significant differences in the clinical outcomes between the groups. These findings support the use of intra-articular injections of expanded MSCs for the treatment of knee OA.
Intra-articular injections of expanded MSCs alone, or in combination with PRP led to improvements of 22.6 and 26.4 points in the KOOS global score at 12 months, respectively. This improvement corresponded to 29% and 31% of the total improvement that was possible. Additionally, the improvements were most significant in the KOOS pain subscore (26.5% and 57.1% for MSCs and MSCs + PRP groups, respectively). Although the MSCs + PRP group showed higher mean percentage improvement, it must be taken into account that at baseline there was a 11.6 points difference favoring the MSCs group, thus suggesting more room for improvement in the MSCs + PRP group. On the other hand, the mean percentage improvement of quality of life subscore showed a tendency to be higher in the MSCs + PRP group (accounting for only 3.4 points difference at baseline), suggesting that the addition of PRP to the MSCs may provide greater overall positive effect which translates into higher quality of life scores.
Despite the lack of high-level studies in the scientific literature, the use of MSCs has been emerging as a potential nonsurgical approach to knee OA [21, 23, 32]. Koh et al. [23] reported 2-year follow-up data on a group patients with knee OA (levels 3 and 4 by the International Cartilage Repair Society Score) who underwent tibial osteotomy and were also treated with concomitant PRP alone (N = 23) or MSCs + PRP (N = 21). They reported significant improvements in the symptoms and pain KOOS subscores at 2 years of follow-up (81.2 and 82.8, respectively) in the MSCs + PRP group when compared to patients that underwent isolated PRP intra-articular injections (74.0 and 75.4, respectively). This study results showed smaller values for pain (MSCs group with 50.8 points and MSCs + PRP group with 75.3 points) and symptoms subscales (MSCs group with 55.1 points and MSCs + PRP group with 63.1 points). A significant limitation of this study is the confounding effect of the concomitant high tibial osteotomy.
The studies reported to date have used intra-articular injections of MSCs alone [21, 23, 36]. In the present study it was used expanded MSCs (40 × 106 cells), which is comparable to the studies reported in the literature (15 × 106 to 40 × 106) [37, 38, 41]. However, comparison to prior studies is difficult due to important differences in cell types used and the lack of rigorous reporting of the exact composition and biologic activity of cell preparations. For example, Vega et al. [38] used allogenic bone marrow-derived MSCs, and the biologic activity of such cells may be affected by an immunological response. Additionally, other studies (uncultured MSCs) have used autologous adipose-derived MSCs [21, 23].
The majority of prior studies reported in the scientific literature have used MSC therapy as a concomitant treatment with other surgical procedures to treat knee OA, including high tibial osteotomy [23, 41], arthroscopic debridement [21, 37], and microfracture [41]. These associated procedures may confound the final outcomes, making it difficult to determine the true effect of the isolated use of MSCs therapy. Additionally, Saw et al. [36] combined the use of MSCs and hyaluronic acid, which may also have confounded the final outcomes. Conversely, in the present study it was used intra-articular injections of MSCs alone or MSCs + PRP without any concomitant treatment in order to assess the isolated effect of this therapy.
Concerns regarding the safety of intra-articular injections of MSCs have been raised in the scientific literature [30]. In the present study, minimal adverse effects were seen in both groups. This finding is in accordance with previous reports in the scientific literature which also demonstrate the safety of MSCs intra-articular injections [12, 33].
There are some limitations to this study. The primary limitation is the small sample size. This study goal was to perform a pilot study to establish the laboratory and clinical research protocols, to allow for further study. The laboratory analyses of CFUs and flow cytometry studies are labor intensive and costly, limiting the number of patients that could be included in this study. Nevertheless, despite the small number of patients, the results obtained from the CFU analysis may be used as reference in future research. Also, this study did not include a control group with a “gold-standard” treatment, such as steroid or hyaluronic acid injection. The lack of a group with intra-articular injections of PRP alone precludes the direct assessment of PRP, i.e., if it acts by potentiating the MSCs effect or if it has a role in directly relieving OA symptoms. Moreover, it included a heterogeneous population in terms of alignment deformity (varus and valgus) and OA severity in order to assess the treatment efficacy in all groups of the disease; however, this may have led to greater variability in the outcomes. Although a 12-month follow-up provides a reasonable insight into the early outcomes, a longer period of follow-up would provide important information concerning the longer-term effects of these therapies and possible deterioration over time. Moreover, the absence of cartilage follow-up imaging evaluation and/or histologic analysis precluded evaluation of the mechanism which led to the reported symptom relief, i.e., it was not possible to state that the symptom relief was due to repair of the damaged cartilage or, more likely, due to an effect on the inflammatory process of the osteoarthritic knee synovium.
This study showed clinical effectiveness and safety of expanded MSCs and the combination of those with PRP for the conservative treatment of symptomatic and radiographic knee OA. Biological strategies are a promising treatment strategy for degenerative disease and its protocols and outcomes must be further studied to achieve better solutions, and to allow avoidance of more invasive surgical approaches. In addition to the symptom relief and functional improvement, regenerative medicine may bring important contributions in the medical decision-making, including the delay for a joint arthroplasty.
Conclusion
The use of intra-articular injections of expanded MSCs alone or in combination with PRP is safe and has a positive effect on symptoms in patients with knee OA. This preliminary data suggest that there are no important clinical outcome differences between both therapies. These results are encouraging and support the recommendation of this minimally invasive procedure in patients with knee OA, without requiring hospitalization.
References
Amable PR, Carias RBV, Teixeira MVT, da Cruz Pacheco Í, do Amaral RJFC., Granjeiro JM et al (2013) Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther 4:67
Bastos Filho R, Magnussen RA, Duthon V, Demey G, Servien E, Granjeiro JM et al (2013) Total knee arthroplasty after high tibial osteotomy: a comparison of opening and closing wedge osteotomy. Int Orthop 37:427–431
Buckwalter JA, Saltzman C, Brown T (2004) The impact of osteoarthritis: implications for research. Clin Orthop Relat Res 427:S6-S15
Campbell KA, Saltzman BM, Mascarenhas R, Khair MM, Verma NN, Bach BR et al (2015) Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy 31:2213–2221
Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213:341–347
Caplan AI (1991) Mesenchymal stem cells. J Orthop Res 9:641–650
Carstairs A, Genever P (2014) Stem cell treatment for musculoskeletal disease. Curr Opin Pharmacol 16:1–6
da Silva Meirelles L, Sand TT, Harman RJ, Lennon DP, Caplan AI (2008) MSC frequency correlates with blood vessel density in equine adipose tissue. Tissue Eng Part A 15:221–229
Dejour H, Carret J, Walch G (1991) Les Gonarthroses. 7émes Journées Lyonnaises de Chirurgie de Genou. 7émes Journées Lyonnaises de Chirurgie de Genou, Les Gonarthroses
do Amaral RJFC., da Silva NP, Haddad NF, Lopes LS, Ferreira FD, Cappelletti PA et al (2016) Platelet-rich plasma obtained with different anticoagulants and their effect on platelet numbers and mesenchymal stromal cells behavior in vitro. Stem Cells Int. https://doi.org/10.1155/2016/7414036
Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA (2009) Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 37:2259–2272
Freitag J, Bates D, Boyd R, Shah K, Barnard A, Huguenin L et al (2016) Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy—a review. BMC Musculoskelet Disord 17:230
Gomoll A, Filardo G, De Girolamo L, Esprequeira-Mendes J, Marcacci M, Rodkey W et al (2012) Surgical treatment for early osteoarthritis. Part I: cartilage repair procedures. Knee Surg Sports Traumatol Arthrosc 20:450–466
Goncalves R, Cabri J, Pinheiro J, Ferreira P, Gil J (2010) Reliability, validity and responsiveness of the Portuguese version of the Knee injury and Osteoarthritis Outcome Score—Physical Function Short-form (KOOS-PS). Osteoarthritis Cartilage 18:372–376
Goyal D, Keyhani S, Lee EH, Hui JHP (2013) Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy 29:1579–1588
Haas DA, Kaplan RS (2017) Variation in the cost of care for primary total knee arthroplasties. Arthroplast Today 3:33–37
Heijink A, Gomoll AH, Madry H, Drobnič M, Filardo G, Espregueira-Mendes J et al (2012) Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 20:423–435
Ishiguro N, Kojima T, Poole AR (2002) Mechanism of cartilage destruction in osteoarthritis. Nagoya J Med Sci 65:73–84
Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC et al (2014) Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem cells 32:1254–1266
Khoshbin A, Leroux T, Wasserstein D, Marks P, Theodoropoulos J, Ogilvie-Harris D et al (2013) The efficacy of platelet-rich plasma in the treatment of symptomatic knee osteoarthritis: a systematic review with quantitative synthesis. Arthroscopy 29:2037–2048
Koh Y-G, Choi Y-J (2012) Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 19:902–907
Koh Y-G, Jo S-B, Kwon O-R, Suh D-S, Lee S-W, Park S-H et al (2013) Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy 29:748–755
Koh Y-G, Kwon O-R, Kim Y-S, Choi Y-J (2014) Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: a prospective study. Arthroscopy 30:1453–1460
Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA et al (2008) Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum 58:26–35
Lohmander LS, Roos EM (2007) Clinical update: treating osteoarthritis. Lancet 370:2082–2084
Mazor M, Lespessailles E, Coursier R, Daniellou R, Best T, Toumi H (2014) Mesenchymal stem-cell potential in cartilage repair: an update. J Cell Mol Med 18:2340–2350
Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD (2016) Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy 32:495–505
Mifune Y, Matsumoto T, Takayama K, Ota S, Li H, Meszaros LB et al (2013) The effect of platelet-rich plasma on the regenerative therapy of muscle derived stem cells for articular cartilage repair. Osteoarthritis Cartilage 21:175–185
Mishra A, Tummala P, King A, Lee B, Kraus M, Tse V et al (2009) Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods 15:431–435
Osborne H, Anderson L, Burt P, Young M, Gerrard D (2015) Australasian College of Sports Physicians—position statement: the place of mesenchymal stem/stromal cell therapies in sport and exercise medicine. Br J Sports Med 50:1237–1244
Owen M, Friedenstein A (1988) Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp 136:42–60
Pas HI, Winters M, Haisma HJ, Koenis MJ, Tol JL, Moen MH (2017) Stem cell injections in knee osteoarthritis: a systematic review of the literature. Br J Sports Med 51:1125–1133
Peeters C, Leijs MJ, Reijman M, van Osch G, Bos P (2013) Safety of intra-articular cell-therapy with culture-expanded stem cells in humans: a systematic literature review. Osteoarthritis Cartilage 21:1465–1473
Pers Y-M, Ruiz M, Noël D, Jorgensen C (2015) Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage 23:2027–2035
Rubio-Azpeitia E, Andia I (2014) Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Musc Ligaments Tendons J 4:52–62
Saw K-Y, Anz A, Jee CS-Y, Merican S, Ng RC-S, Roohi SA et al (2013) Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy 29:684–694
Tan Y, Jiang M, Yu H, Li J, Qing Z (2013) Therapeutic effect of arthroscopy combined with autologous bone marrow stem cell grafting on knee osteoarthritis. J Tradit Chin Orthop Traumatol 10:35–38
Vega A, Martín-Ferrero MA, Del Canto F, Alberca M, García V, Munar A et al (2015) Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation 99:1681–1690
Vinatier C, Mrugala D, Jorgensen C, Guicheux J, Noël D (2009) Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol 27:307–314
Wolfstadt JI, Cole BJ, Ogilvie-Harris DJ, Viswanathan S, Chahal J (2015) Current concepts: the role of mesenchymal stem cells in the management of knee osteoarthritis. Sports Health 7:38–44
Wong KL, Lee KBL, Tai BC, Law P, Lee EH, Hui JH (2013) Injectable cultured bone marrow—derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy 29:2020–2028
Xia P, Wang X, Lin Q, Li X (2015) Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: a systematic review and meta-analysis. Int Orthop 39:2363–2372
Xie X, Wang Y, Zhao C, Guo S, Liu S, Jia W et al (2012) Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials 33:7008–7018
Zhang W, Moskowitz R, Nuki G, Abramson S, Altman R, Arden N et al (2008) OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 16:137–162
Funding
This study was funded by ESHO Empresa de Serviços Hospitalares S.A. (Grant number: not applicable).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that this study received funding from ESHO Empresa de Serviços Hospitalares S.A. Ricardo Bastos received grants from ESHO Empresa de Serviços Hospitalares S.A. The others authors declare no conflict of interest.
Ethical approval
The research was approved by the Comissão Nacional de Ética em Pesquisa (Brazilian National Ethics in Research Committee)—CONEP, under number 14878813.4.0000.5533 and was conducted in accordance with the principles of the Declaration of Helsinki.
Informed consent
All the participants signed the written consent form before inclusion in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bastos, R., Mathias, M., Andrade, R. et al. Intra-articular injections of expanded mesenchymal stem cells with and without addition of platelet-rich plasma are safe and effective for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 26, 3342–3350 (2018). https://doi.org/10.1007/s00167-018-4883-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-018-4883-9