Abstract
Purpose
This meta-analysis compared infection and revision rates in patients with rheumatoid arthritis (RA) and osteoarthritis (OA) who underwent total knee arthroplasty (TKA). Rates of superficial wound and deep periprosthetic infections were compared in the groups, as were whether revision rates associated with infectious and noninfectious causes differed in the RA and OA groups.
Methods
Studies were included in the meta-analysis if they (1) compared infection and revision rates after primary TKA in RA and OA patients; (2) directly compared superficial wound and deep periprosthetic infection rates in RA and OA patients who underwent primary TKA; and (3) reported the actual numbers of RA and OA patients who underwent TKA and developed postoperative infection and/or required revision.
Results
The rate of superficial wound infections after primary TKA was similar in the RA and OA groups (15/258 [5.8 %] vs. 77/1609 [4.7 %]; odds ratio [OR] 1.12, 95 % confidence interval [CI] 0.36–3.46; P = n.n.), but the deep infection rate was significantly higher in RA than in OA patients (229/7651 [3.0 %] vs. 642/68628 [0.9 %]; OR 2.04, 95 % CI 1.37–3.05; P < 0.001). The proportion of subjects who required revision resulting from infection after TKA was significantly higher in the RA than in the OA group (86/8201 [1.0 %] vs. 555/118755 [0.5 %]; OR 1.89, 95 % CI 1,34–2.66; P < 0.001), whereas the proportion of subjects requiring revision due to noninfectious causes did not differ significantly (46/594 [7.7 %] vs. 52/904 [5.7 %]; OR 1.22, 95 % CI 0.74–2.00; P = n.n.)
Conclusion
Following primary TKA, RA patients had a significantly higher rate of deep periprosthetic infections than OA patients, but their superficial infection rates were similar. The revision rate due to infectious causes was significantly higher in RA than in OA patients, but their revision rates due to noninfectious causes did not differ. Therefore, the surgeon should fully explain to RA patients scheduled to undergo primary TKA that, compared to OA patients, they are more likely to experience a deep infection postsurgery.
Level of evidence
Meta-analysis Level III.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The long-term prognosis of patients with rheumatoid arthritis (RA) has improved due to the development of new medications, such as disease-modifying anti-rheumatic drugs (DMARDs) and biologic agents [10, 14]. Nevertheless, approximately 18–24 % of RA patients still progress to end-stage arthritis and require arthroplasty [8, 13], with the knee joint being the most frequent site of arthroplasty in RA patients [31]. The number of RA patients undergoing total knee arthroplasty (TKA) has increased [18], although the vast majority of patients who undergo TKA do so for advanced knee osteoarthritis (OA) [6, 16]. Surgical results of TKA have been regarded as poorer in RA than in OA patients, due to the high infection and revision rates resulting from the characteristics of RA, such as chronic inflammation [3, 24, 25, 27]. To date, however, few studies have directly compared infection and/or revision rates in patients with RA and OA, with some of these comparative studies showing contradictory results [17]. Comparisons have also been hampered by unclear definitions of infection, by whether superficial wounds or deep periprosthetic infections were being compared, and by whether revisions are or are not infection related.
This meta-analysis was therefore designed to better compare the surgical outcomes of TKA for RA and OA, including infection and revision rates. Subgroup analyses were used to compare the rates of superficial wound and deep periprosthetic infections in the two groups, and to determine whether the revision rates due to infectious and noninfectious causes differed between the RA and OA groups.
Materials and methods
Data and literature sources
This study was based on Cochrane review methods. Multiple comprehensive databases, including MEDLINE, EMBASE, Web of Science, SCOPUS, and the Cochrane Library (January 1, 1987– June 30, 2015), were searched for studies that compared superficial and/or deep infection rates and revision rates resulting from infectious and/or noninfectious causes following primary TKA in RA and OA patients. There were no restrictions on language or year of publication. Search terms used in the title, abstract, MeSH, and keywords fields included “arthroplasty” [tiab] OR “replacements” [tiab] OR “knee” [tiab], and “rheumatoid arthritis” [MeSH] OR “osteoarthritis” [MeSH] OR “infection” [tiab] OR “revision” [tiab]. After the initial electronic search, relevant articles and their bibliographies were searched manually. The identified articles were individually assessed for inclusion.
Study selection
From the title and abstract, two reviewers independently selected the relevant studies for full review. The full text of an article was reviewed if the abstract did not provide sufficient data to make a decision. Studies were included in the meta-analysis if they (1) compared infection and revision rates after primary TKA in RA and OA patients; (2) directly compared superficial wound and deep periprosthetic infection rates and their associated revision rates in RA and OA patients who underwent primary TKA; and (3) reported the actual numbers of total RA and OA patients who underwent TKA and the actual numbers who developed postoperative infection and/or required revision. Studies were excluded if they did not clearly distinguish between superficial and deep infections or did not clearly determine the cause of revision, whether infectious or noninfectious.
Data extraction
Two reviewers independently recorded data from each study using a predefined data extraction form. Disagreements between the reviewers were resolved by consensus or by discussion with a third investigator when a consensus could not be reached. Variables recorded included those associated with assessing infection and revision rates: (1) the numbers of patients with RA or OA who developed superficial wound and/or deep periprosthetic infections following primary TKA; (2) the number of revisions due to infectious and noninfectious causes after primary TKA in the RA and OA groups; and (3) the sample size of each group. If these variables were not mentioned in the articles, the study authors were contacted to retrieve further information. Superficial and deep infections were defined based mainly on Center for Disease Control (CDC) Criteria as follows: superficial infection does not require reoperation and is cured by antibiotics alone, whereas deep infection requires additional surgery such as open or arthroscopic debridement, or two-stage revision [7].
Assessment of methodological quality
Two reviewers independently assessed the methodological qualities of each study using the Newcastle-Ottawa Scale, as recommended by the Cochrane Non-Randomized Studies Methods Working Group. For the purposes of the current meta-analysis, the adjusted Newcastle-Ottawa Scale star system was used. The Newcastle-Ottawa Scale had three criteria: the selection of the study groups; the comparability of the groups; and, for case–control and cohort studies, the determination of exposure or outcome of interest. Studies of high quality were defined as those having scores >5 points. Disagreements in scores were resolved by discussion and consensus between the two reviewers.
Data synthesis and statistical analysis
The main outcomes of the meta-analysis were the proportions of patients in the RA and OA groups who underwent TKA and experienced superficial and deep infections and the proportions of patients requiring revision due to infectious and noninfectious causes. The odds ratios (ORs) and 95 % confidence intervals (CI) of binary outcomes were calculated for all comparisons. These values were analysed with a random effects model. Interrater reliability in assessing methodological quality was evaluated by kappa (к), with values of ≤0.40, 0.41–0.60, 0.61–0.80, and 0.81–1.00 indicating no, moderate, substantial, and almost perfect agreement, respectively. Heterogeneity was determined by estimating the proportion of between-study inconsistencies due to actual differences between studies, rather than differences due to random error or chance, using I2 statistics, with 25, 50, and 75 % defined as low, moderate, and high heterogeneity, respectively. All statistical analyses were performed with RevMan version 5.2 statistics software.
Results
Identification of studies
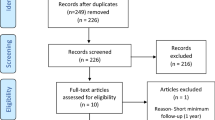
Figure 1 shows details of study identification, inclusion, and exclusion. An electronic search yielded 2990 studies in PubMed (MEDLINE), 3328 in EMBASE, 692 in Web of Science, 4598 in SCOPUS, and 248 in the Cochrane Library. Two additional publications were identified through manual searching. After 4905 duplicates were removed, 6953 studies remained. Of these, 6894 were excluded as it was clear from their abstracts and titles that they did not fulfil the selection criteria. An additional 46 studies were excluded because they did not provide usable information, did not differentiate between superficial and deep infection, or did not clearly describe cause of revision. Thus, 13 studies [1, 5, 7, 9, 11, 12, 17, 19–21, 25, 28, 30] were included in this meta-analysis.
Study characteristics and quality of the included studies
Of the 13 included studies, five compared infection rate, including superficial and/or deep infection rate, between RA and OA groups; six compared revision rate due to infectious and noninfectious causes, and two compared both infection and revision rates between RA and OA groups (Table 1).
All 13 studies were at low risk of selection bias. All provided detailed demographic data of each RA and OA group. None assessed possible confounding factors. Of these 13 studies, eight were considered high quality, with >5 points on the Newcastle-Ottawa Scale. Interrater reliabilities (к values) for all items of NOS ranged from 0.71 to 0.89, indicating at least more than substantial agreement between two investigators.
Superficial versus deep infection
Of the 13 studies, three reported rates of superficial infection; six reported rates of deep prosthetic infection; and three reported total infection rates, including both superficial and deep infection rates, after in RA and OA patients who underwent primary TKA. The rate of superficial wound infections after primary TKA was similar in the RA and OA groups (15/258 [5.8 %] vs. 77/1609 [4.7 %]; OR 1.12, 95 % CI 0.36–3.46; P = n.n.). In contrast, the rate of deep prosthetic infection was significantly higher in RA than in OA patients (229/7651 [3.0 %] vs. 642/68628 [0.9 %]; OR 2.04, 95 % CI 1.37–3.05; P < 0.001, Fig. 2).
Forest plot showing infection rates in patients with rheumatoid arthritis (RA) and osteoarthritis (OA). The rates of superficial wound infections after primary TKA were similar in the RA and OA groups (15/258 [5.8 %] vs. 77/1609 [4.7 %]; OR 1.12, 95 % CI 0.36–3.46; P = n.s.). In contrast, the rate of deep periprosthetic infections was significantly higher in RA than in OA patients (229/7651 [3.0 %] vs. 642/68628 [0.9 %]; OR 2.04, 95 % CI 1.37–3.05; P < 0.001)
Infectious versus noninfectious causes of revision
Of the 13 studies, seven and five reported rates of revision due to infectious and noninfectious causes, respectively, in RA and OA patients who underwent primary TKA. The rate of revision due to infectious causes was significantly higher in the RA than in the OA group (86/8201 [1.0 %] vs. 555/118755 [0.5 %]; OR 1.89, 95 % CI 1, 34–2.66; P < 0.001), whereas the rate of revision due to noninfectious causes was similar in these two groups (46/594 [7.7 %] vs. 52/904 [5.7 %]; OR 1.22, 95 % CI 0.74–2.00; P = n.n., Fig. 3).
Forest plot showing revision rates in patients with rheumatoid arthritis (RA) and osteoarthritis (OA). The rate of revision due to infection was significantly higher in RA than in OA patients (86/8201 [1.0 %] vs. 555/118755 [0.5 %]; OR 1.89, 95 % CI 1, 34–2.66; P < 0.001). In contrast, the rates of revision due to noninfectious causes were similar in the two groups (46/594 [7.7 %] vs. 52/904 [5.7 %]; OR 1.22, 95 % CI 0.74–2.00; P = n.n.)
Meta-regression analyses
The results of meta-regression analyses are reported in Table 2. Age and gender were not significantly associated with differences in infection and revision rates after primary TKA in the RA and OA groups. This finding indicated that the results of the current meta-analysis were not biased by the demographic characteristics of the patients in the included studies. Another meta-regression analysis was performed to evaluate the confounding effect of follow-up period on differences in superficial or deep infection rates between RA and OA patients. The results showed no significant association between follow-up duration and differences in superficial or deep infection rates, indicating that follow-up duration did not influence the differences in superficial or deep infection rates between RA and OA patients.
Discussion
The most important findings of this meta-analysis were that the rates of deep, but not superficial, infections and the rates of revision due to infectious, but not noninfectious, causes were significantly higher in patients who underwent primary TKA for RA than for OA.
Infections after TKA can be classified as superficial wound and deep periprosthetic infections. Distinguishing between these two categories is important because their treatment strategies differ. Superficial infections can usually be controlled by antibiotic therapy, whereas deep infections usually require prosthesis removal followed by second stage revision. Despite the importance of knowing the rates of superficial and deep infections, several previous studies [24, 27] comparing infection rates after TKA for RA and OA did not distinguish between superficial and deep infections. This meta-analysis may therefore be meaningful in that it included subgroup analysis of superficial and deep infection rates after TKA for RA and OA.
Although the causes of the higher deep prosthesis infection rate in RA than in OA patients have not yet been determined, immunosuppressive treatment of RA patients with corticosteroids and/or immunomodulatory drugs may be associated with deep prosthesis infection [21, 24, 27]. Similarly, allogenic transfusion may increase the deep infection rate in these patients. Many patients with RA experience anaemia due to bone marrow suppression by chronic disease and medication [26]. These patients are more likely susceptible to postoperative anaemia, increasing the need for allogenic blood transfusion, which can increase the risk of postoperative infection by inducing transfusion-related immunomodulation [2, 4, 23]. It is therefore unclear whether RA itself or its treatment, including immunomodulation and allogenic transfusion, increases the risk for deep prosthesis infection after surgery.
This meta-analysis, however, found no significant difference in rates of superficial infection between RA and OA patients. Although immunosuppressive factors such as RA medications and transfusion can affect both superficial and deep infections, it is unclear why deep, but not superficial, infection rates differed between the RA and OA groups. Perioperative antibiotics may prevent or cure superficial wound infections, even in immunosuppressed patients, such as those with RA. However, once a biofilm forms around a prosthesis (deep periprosthetic infection), it may be more difficult for antibiotics to eliminate the bacteria, even those with low virulence, in immunosuppressed RA than in OA patients [32]. These differences in the effectiveness of antibiotics for superficial and deep infections in RA, but not in OA, patients may contribute to their similar superficial infection rates, along with a higher deep infection rate in RA than in OA patients.
The current meta-analysis also showed that the revision rate due to infectious causes was higher in RA than in OA patients, but there were no significant between-group differences in revision rates due to noninfectious causes. This finding indicated that the main cause of revision in RA patients was infection, not complications due to chronic inflammation, such as soft tissue laxity or attenuation of the posterior cruciate ligament. These latter conditions were regarded as important concerns in RA patients undergoing primary TKA. Prosthesis instability was reported to be more common in cruciate retaining (CR) than in posterior substitution (PS)-type prostheses. This result has been attributed to joint laxity and PCL attenuation caused by chronic inflammation [15]. However, recent increases in the use of PS than of CR-type TKA may reduce revision rates due to noninfectious causes, including prosthesis instability [29]. The similar revision rates due to noninfectious causes may also be due to the relative lower activity level in RA than in OA patients [22, 26, 27].
This study had several limitations. The possibility of other confounding factors affecting the results could not be completely ruled out, especially as age distributions differed between the RA and OA groups. Different age distributions in the two groups may have influenced the superficial infection and revision rates by underestimating the real incidence of infection and revision in the RA group, because patients in that group were younger and tended to be healthier compared to the group of patients with OA. However, the meta-regression analysis performed in our study showed that age and gender were not significantly associated with differences in the rates of infection and revision in RA and OA patients, thereby excluding any confounding effect of age distribution on infection and revision rates after TKA in the two groups. The present study sought to analyse other confounding factors such as BMI, surgical time and techniques, and institutional perioperative care. Moreover, there may have been inconsistencies among the included studies in terms of the diagnosis criteria used for infection after TKA, for example whether the infection was diagnosed based on clinical decisions or culture results from aspiration or intraoperative tissue specimen; this may have affected the difference in infection rate. However, due to a lack of information for these confounding factors in each study, meta-regression with the above factors could not be performed.
Another limitation may have been potential bias stemming from unmeasured or unknown confounders, including medications and disease severity in patients with RA. Lastly, it would be ideal to perform a meta-analysis including only randomized control trials (RCTs). However, most studies in the orthopaedic field are not RCTs, because most deal with surgical results. Therefore, although the current meta-analysis included non-RCT studies comparing the infection and revision rate between RA and OA patients, we believe that it constitutes an examination of the best evidence currently available.
Conclusions
The deep periprosthetic infection rate after primary TKA was higher in RA than in OA patients, whereas the superficial wound infection rate was similar in the two groups. Revision rates caused by soft tissue laxity or PCL attenuation following primary TKA were also similar in RA and OA patients. In contrast, the revision rate due to periprosthetic infection was significantly higher in RA than in OA patients.
References
Bengtson S, Knutson K (1991) The infected knee arthroplasty. A 6-year follow-up of 357 cases. Acta Orthop Scand 62:301–311
Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB (1999) An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am 81:2–10
Bongartz T, Halligan CS, Osmon DR, Reinalda MS, Bamlet WR, Crowson CS, Hanssen AD, Matteson EL (2008) Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum 59:1713–1720
Carson JL, Altman DG, Duff A, Noveck H, Weinstein MP, Sonnenberg FA, Hudson JI, Provenzano G (1999) Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion (Paris) 39:694–700
Chesney D, Sales J, Elton R, Brenkel IJ (2008) Infection after knee arthroplasty a prospective study of 1509 cases. J Arthroplasty 23:355–359
Choi YJ, Ra HJ (2016) Patient satisfaction after total knee arthroplasty. Knee Surg Relat Res 28:1–15
da Cunha BM, de Oliveira SB, Santos-Neto L (2011) Incidence of infectious complications in hip and knee arthroplasties in rheumatoid arthritis and osteoarthritis patients. Rev Bras Reumatol 51:609–615
da Silva E, Doran MF, Crowson CS, O’Fallon WM, Matteson EL (2003) Declining use of orthopedic surgery in patients with rheumatoid arthritis? Results of a long-term, population-based assessment. Arthritis Rheum 49:216–220
Elke R, Meier G, Warnke K, Morscher E (1995) Outcome analysis of total knee-replacements in patients with rheumatoid arthritis versus osteoarthritis. Arch Orthop Trauma Surg 114:330–334
Emery P (2002) Evidence supporting the benefit of early intervention in rheumatoid arthritis. J Rheumatol Suppl 66:3–8
Goldberg VM (2001) Principles of revision total knee arthroplasty: overview. Instr Course Lect 50:357–358
Jamsen E, Huhtala H, Puolakka T, Moilanen T (2009) Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am 91:38–47
Kapetanovic MC, Lindqvist E, Saxne T, Eberhardt K (2008) Orthopaedic surgery in patients with rheumatoid arthritis over 20 years: prevalence and predictive factors of large joint replacement. Ann Rheum Dis 67:1412–1416
Korpela M, Laasonen L, Hannonen P, Kautiainen H, Leirisalo-Repo M, Hakala M, Paimela L, Blafield H, Puolakka K, Mottonen T, Group FI-RT (2004) Retardation of joint damage in patients with early rheumatoid arthritis by initial aggressive treatment with disease-modifying antirheumatic drugs: five-year experience from the FIN-RACo study. Arthritis Rheum 50:2072–2081
Laskin RS, O’Flynn HM (1997) The Insall Award. Total knee replacement with posterior cruciate ligament retention in rheumatoid arthritis. Problems and complications. Clin Orthop Relat Res 345:24–28
Lee BJ, Kyung HS, Yoon SD (2015) Two-stage revision for infected total knee arthroplasty: based on autoclaving the recycled femoral component and intraoperative molding using antibiotic-impregnated cement on the tibial side. Clin Orthop Surg 7:310–317
LoVerde ZJ, Mandl LA, Johnson BK, Figgie MP, Boettner F, Lee YY, Goodman SM (2015) Rheumatoid arthritis does not increase risk of short-term adverse events after total knee arthroplasty: a retrospective case-control study. J Rheumatol 42:1123–1130
Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA (2014) US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol 66:1432–1439
Nafei A, Kristensen O, Knudsen HM, Hvid I, Jensen J (1996) Survivorship analysis of cemented total condylar knee arthroplasty. A long-term follow-up report on 348 cases. J Arthroplasty 11:7–10
Partio E, Orava T, Lehto MU, Lindholm ST (1994) Survival of the Townley knee. 360 cases with 8 (0.1-15) years’ follow-up. Acta Orthop Scand 65:319–322
Ravi B, Croxford R, Hollands S, Paterson JM, Bogoch E, Kreder H, Hawker GA (2014) Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol 66:254–263
Ravi B, Escott B, Shah PS, Jenkinson R, Chahal J, Bogoch E, Kreder H, Hawker G (2012) A systematic review and meta-analysis comparing complications following total joint arthroplasty for rheumatoid arthritis versus for osteoarthritis. Arthritis Rheum 64:3839–3849
Rosencher N, Kerkkamp HE, Macheras G, Munuera LM, Menichella G, Barton DM, Cremers S, Abraham IL, Investigation O (2003) Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion (Paris) 43:459–469
Schnaser EA, Browne JA, Padgett DE, Figgie MP, D’Apuzzo MR (2015) Perioperative complications in patients with inflammatory arthropathy undergoing total knee arthroplasty. J Arthroplasty 30:76–80
Schrama JC, Espehaug B, Hallan G, Engesaeter LB, Furnes O, Havelin LI, Fevang BT (2010) Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res (Hoboken) 62:473–479
Singh JA, Inacio MC, Namba RS, Paxton EW (2015) Rheumatoid arthritis is associated with higher ninety-day hospital readmission rates compared to osteoarthritis after hip or knee arthroplasty: a cohort study. Arthritis Care Res (Hoboken) 67:718–724
Stundner O, Danninger T, Chiu YL, Sun X, Goodman SM, Russell LA, Figgie M, Mazumdar M, Memtsoudis SG (2014) Rheumatoid arthritis vs osteoarthritis in patients receiving total knee arthroplasty: perioperative outcomes. J Arthroplasty 29:308–313
Tayton ER, Frampton C, Hooper GJ, Young SW (2016) The impact of patient and surgical factors on the rate of infection after primary total knee arthroplasty: an analysis of 64 566 joints from the New Zealand Joint Registry. Bone Joint J 98-B:334–340
Watanabe T, Muneta T, Koga H, Horie M, Nakamura T, Otabe K, Nakagawa Y, Katakura M, Sekiya I (2016) In-vivo kinematics of high-flex posterior-stabilized total knee prosthesis designed for Asian populations. Int Orthop. doi:10.1007/s00264-016-3176-5
Weir DJ, Moran CG, Pinder IM (1996) Kinematic condylar total knee arthroplasty. 14-year survivorship analysis of 208 consecutive cases. J Bone Joint Surg Br 78:907–911
Wolfe F, Zwillich SH (1998) The long-term outcomes of rheumatoid arthritis: a 23-year prospective, longitudinal study of total joint replacement and its predictors in 1,600 patients with rheumatoid arthritis. Arthritis Rheum 41:1072–1082
Zurcher-Pfund L, Uckay I, Legout L, Gamulin A, Vaudaux P, Peter R (2013) Pathogen-driven decision for implant retention in the management of infected total knee prostheses. Int Orthop 37:1471–1475
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
No funds were received to do this research.
Ethical approval
There were no ethical approval, because this study was a meta-analysis based on the data of previously published studies.
Informed consent
Informed consent was not applicable since the study design was a meta-analysis.
Additional information
Do-Kyung Lee and Hyun-Jung Kim authors are contributed equally to the work.
Rights and permissions
About this article
Cite this article
Lee, DK., Kim, HJ., Cho, IY. et al. Infection and revision rates following primary total knee arthroplasty in patients with rheumatoid arthritis versus osteoarthritis: a meta-analysis. Knee Surg Sports Traumatol Arthrosc 25, 3800–3807 (2017). https://doi.org/10.1007/s00167-016-4306-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-016-4306-8