Abstract
Purpose
Antibiotic-loaded bone cement has been widely used for the treatment of infected knee replacement, but its routine use in primary TKA remains controversial. The aim of this systematic review was to analyze the literature about the antimicrobial efficacy and safety of antibiotic-loaded bone cement for its prophylactic use in primary TKA.
Methods
A detailed and systematic search of the Pubmed, Medline, Cochrane Reviews and Google Scholar databases had been performed using the keyword “total knee arthroplasty” “total knee replacement” “total knee prosthesis” and “antibiotic-loaded bone cement” with no limit regarding the year of publication. We used modified Coleman scoring methodology (mCMS) to identify scientifically sound articles in a reproducible format. The review was limited to the English-language articles.
Results
Six articles met inclusion criteria. In total, 6318 arthroplasties were included in our study. 3217 of these arthroplasties received antibiotic-loaded bone cement and 3101 arthroplasties served as the control. There was no statistical difference between the two groups in terms of the incidence of deep or superficial surgical site infection. The average mCMS score was 67.6, indicating good methodological quality in the included studies.
Conclusions
Present review did not reveal any significant difference in terms of rate of deep or superficial surgical site infection in patients receiving antibiotic-loaded bone cement compared with the control (plain bone cement) during primary TKA. The clinical relevance of this study was that the use of antibiotic-loaded bone cement did not significantly reduce the risk of infection in primary TKA.
Level of evidence
III.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infection following primary total knee arthroplasty (TKA) is still a serious complication which can result in costly revision surgery, reduction of the patient’s functional status, and prolonged hospitalization [25]. Deep infection is reported as the second most common cause of failure of an implant, with an incidence rate of 1 to 3 % [24]. Intra-articular biomaterials are risk factors for bacterial contamination and subsequent infection. Acrylic bone cement carries a particularly high risk of bacterial colonization compared with other materials such as metal and polyethylene. Systemic antibiotics, which are commonly used to prevent or treat periprosthetic infection associated with arthroplasty, may not be sufficiently effective to avoid deep infection because of impaired blood circulation and low antibiotic concentrations at the implantation site. Therefore, the use of antibiotic-loaded bone cement (ALBC) is a logical prophylactic measure that has proved effective in treating established infection in revision knee arthroplasty.
Since its introduction by Buchholz and Engelbrecht in 1970, in the form of spacers or beads, ALBC has been commonly used for treating established infection in revision TKA, rather than for infection prophylaxis in primary TKA [3]. ALBCs are commonly classified as “low dose” (<or = 2 g of antibiotic per 40 g of cement), generally used for prophylaxis, and “high-dose” (>2 g antibiotic per 40 g of cement), used to treat infected joint replacements [2, 10, 11, 19]. The delivery of antibiotic from the cement begins immediately after implantation, with the greatest bioavailability occurring within the first 9 weeks after implantation [17]. Several premixed formulations that combine bone cement with various concentrations of different antibiotics, such as penicillin, gentamycin, erythromycin, cephalosporins, tobramycin, vancomycin, cefuroxime, oxacillin and colistin, are commercially available [19]. The antibiotic more frequently used is gentamycin, by virtue of its broad-spectrum bactericidal effect, its stability at high temperatures and the low incidence of allergic reactions [6, 19].
Some authors are against the routine use of ALBC for primary TKA. First, because of the possible risk of hypersensitivity or toxicity [1]. Additionally, there could be a reduction in the mechanical properties of the cement, although this is probably negligible if the antibiotic is used in low doses, not more than 1 g per 40 g of cement. Indeed, biomechanical testing has shown that, in contrast to the use of high-dose antibiotics which can weaken bone cement, the low-dose ALBC shows negligible reductions in fatigue strength, so implant fixation is not compromised [13]. Another main concern is related to the increased cost, which could be overlooked reducing the incidence of peri-prosthetic infections [8]. Finally, there could be so a risk of selection of antibiotic-resistant strains of bacteria [13].
In some European countries, such as the United Kingdom [15], Sweden [20], Denmark and Norway [19], the use of ALBCs in primary TKA has been a standard and common practice for many years, even though the scientific background for their use is uncertain. Conversely, ALBCs are much less frequently used in other countries (the United States [14] and other European countries, including Spain, Poland and Russia [19]).
The worldwide use of ALBC during primary TKA continues to increase although its effectiveness in reducing infection is not yet universally accepted and demonstrated. Therefore, a comprehensive literature search was performed to prove whether the ALBC use during primary TKA could reduce the rate of surgical-site infections, including superficial and deep infections.
Materials and methods
A systematic review of the available literature was performed using the keyword terms “total knee arthroplasty”, “total knee replacement”, “total knee prosthesis” and “antibiotic-loaded bone cement”; there was no limit on the year of publication. The search was limited to English papers. Studies in other languages were not included in this review. The following databases were accessed on 9th April 2016: PubMed (http://www.ncbi.nlm.nih.gov/sites/entrez/); Medline on Ovid (http://www.ovid.com); Cochrane Reviews (http://www.cochrane.org/reviews/), Google Scholar (http://scholar.google.com/). All peer-reviewed journals were considered, and randomized controlled trials (RCTs), prospective (PRO) trials and retrospective (RE) studies were included. Two authors (KC and MG) independently screened the titles and abstracts of the articles resulting from the searches. If the abstract was not available, the authors reviewed the whole document so as to avoid search bias. They also hand searched conference proceedings dealing with the specific topic, and reviewed reference lists and author lists of all papers. In the event of disagreement, a consensus was reached by discussion, if necessary with the intervention of the senior author (ASP).
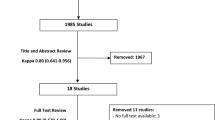
The article selection process was performed according to the Prisma 2015 flow chart (http://www.prisma-statement.org/) [21]. Of 260 articles initially identified, 235 were rejected on the basis that the title and abstract were irrelevant. The full papers of the remaining 25 studies were retrieved and 11 articles were subsequently excluded because the studies did not involve primary TKA or were not RCTs, RE studies or PRO trials. A further five studies were then excluded on the basis of other inclusion and exclusion criteria. Finally six articles met the inclusion criteria. Figure 1 shows the flow chart of the study selection process.
Inclusion and exclusion criteria
In order to be considered eligible for inclusion, studies needed to: (1) include patients undergoing a primary TKA; (2) include an ALBC trial group and a control group whose treatment involved the use of plain bone cement (PBC), irrespective of the dose of administration; (3) be a published RCT, RE study or PRO trial. Studies were excluded if: (1) outcomes of ALBC use in primary TKA were not reported; (2) it was impossible to extrapolate or calculate the necessary data from the published results; (3) primary study patients were in poor physical health, e.g. affected by diseases such as diabetes, malignant tumor; (4) they were animal experiments, in vitro trials or concerned revision arthroplasty, and the operated joint was not the knee, or knee and hip.
Methodological quality assessment
Two investigators (KC and MG) separately evaluated the methods reported in the selected studies, applying the modified Coleman methodology score (mCMS), which takes into account ten criteria assessing methodological quality. Each study was assessed for each of the ten criteria to obtain a final score ranging from 0 to 100. A perfect score of 100 would represent a study design that largely avoids the influence of chance, various biases, and confounding factors.
Results
Tables 1 and 2 present the demographic characteristics of the cohorts reviewed and a summary of the data they present. The studies analyzed in this systematic review had an average Coleman score of 67.6, which confirms the good methodological quality of the available literature. The results did not show lower rates of deep and superficial infections in ALBC group than those in the PBL group, so the use of ALBC in primary TKA could not reduce the infection rate. Only two studies [4, 6] showed a significant effect of ALBC in preventing deep infection in primary TKA. Instead of, contradictory results were reported in two RE [16, 26], in a PRO [7] and in a RCT [12].
In total, 6318 arthroplasties were included in this review; ALBC was used in 3217 of these arthroplasties, while the other 3101 served as the controls (Table 3). Of the six analyzed studies, two were from European countries (France and Spain), one was from Canada, one from the USA, and two from China. Although strict inclusion criteria were used, some aspects of the studies differed, such as the choice of surgical technique, type of implant and bone cement, antibiotics and their dosage. Five studies reported the type of antibiotic and the method for mixing the antibiotic in the cement. One study [12] reported the use of hand-mix preparation including erythromycin and colistin. Other four studies indicated the use of premixed low dose ALBC preparations (cefuroxime, gentamycin, tobramycin) [4, 6, 7, 26]. Only four studies indicated the quantity of antibiotic (2 g, 1 g, 0.5 g/3 milion units and 0.5 g) [4, 7, 12, 26] used in 40 g of bone cement. All the studies provided relevant information about deep infection and superficial infection. Three studies included patient to high risk [12, 16, 26]. The allocation concealment procedures in the six eligible studies were unclear. Only two of these six reports included adequate blinding procedures. The total length of follow-up was variable, ranging from 12 to 49 months. No side effect after the application of ALBC was reported in any of the studies analized.
Discussion
The most important finding of the present study was that use of ALBC in primary TKA did not significantly reduce rate of deep or superficial surgical-site infection.
Many authors have recommended the use of ALBC in primary TKA for infection prophylaxis [4, 6, 19] but data from National Registries, RCTs and meta-analysis studies seem to indicate that ALBC exerts a protective effect against infection only when used in hips [23]. Although ALBC is worldwide used in primary TKA procedures, especially in some Northern European countries, its prophylactic effect against deep infection remains controversial. The percentage of surgeons who routinely use ALBC in primary TKA is >90 % in some countries, such as the United Kingdom, Norway and Sweden, compared with approximately 10 % in other countries, such as the United States.
Chiu et al. [4] were the first to report that cefuroxime-impregnated cement could reduce the deep infection rate from 3.1 % (5/162) to 0 % (0/178). They found no infections in the ALBC group and five infections in the PBC group, a difference that was statistically significant (p = 0.0238). It is to be noted that all five infections reported in their study occurred in diabetic patients. When diabetic patients were removed from the study, there was no difference in infection rates between the two groups. In another retrospective cohort study, Eveillard et al. [6] concluded that ALBC could prevent infection in TKA, reporting a p value close to the limit of significance (9.51 vs 1.21 %, p = 0.07). Since 2004, several authors have reported contradictory results. Recently, data from two retrospective cohort studies [16, 26], one prospective trial [7] and one prospective randomized trial [12] supported the conclusion that ALBC could not prevent deep infection after primary TKA. In particular, in a large retrospective community knee registry study, Namba et al. [16] found no difference in the rates of deep infection (p = 0.002) between patients treated with ALBC (1.4 %, 28/2030) and those receiving PBC (0.7 %, 154/20,869). This study did not report the type of ALBC used, the use of ultraclean air, or systemic antibiotic use, and the ALBC group at baseline contained a significantly higher number of diabetics, patients with an American Society of Anestesiologist (ASA) score >3, and no-osteoarthritis diagnoses. Gandhi et al. [7] reached the same conclusion (2.2 vs 3.1 %, p = 0.84). Hinarejos et al. [12] found that the use of erythromycin- and colistin-loaded bone cement did not lead to a decrease in the rate of infection compared with administration of a systemic prophylactic antibiotic (1.37 vs 1.35 %, p = 0.96). Wang et al. [26] compared the rates of deep infection in gentamicyn-loaded versus plain bone showing that the use of ALBC was not predictive of a lower incidence of deep infection at 1 year (p = 0.865).
Although the present authors attempted to perform a well-designed systematic review, this study inevitably presents some inherent limitations, for example the choice of cements, antibiotics and the method of preparation were not standardized. Firstly, the primary TKAs were performed with Palacos R (Zimmer Holding Inc., Warsaw, IN), Simplex P (Stryker Orthopaedic, Mahwah, NJ), or SmartSet HV (DePuy Orthopaedics, Warsaw, IN. In different types of bone cement there are significant differences in the elution kinetics. Palacos bone cement generally is considered to have the most favourable elution kinetics compared with CMW or Simplex P bone cement [1]. Additionally, the type and the dosage of antibiotic in the cement varied in different studies. The antibiotic must have a broad antibacterial spectrum (including gram positive and gram negative bacteria) and a low percentage of resistant species. Gentamycin has been the antimicrobials most commonly mixed into bone cement in clinical studies worldwide, by virtue of its broad-spectrum bactericidal effect. Its stability at high temperatures and the low incidence of allergic responses [1]. Finally, the method of mixing is considered one of the most important factors that affect the release of the antibiotics and the mechanical properties of cement. The preparation should be as porous as possible in order to increase the spread of the antibiotic, but no excessively porous to weaken the structure of the cement itself. Therefore, the antibiotic destined to be mixed with the cement must be chemically and thermally stable [19]. The manual preparation reduces the strength of cement of 36 % compared to the ALBC prepared industrially. The improvement of mechanical properties due to the greater compactness of the structure of the cement could lead to a decrease in the rate of diffusion of the antibiotic [1].
Another limitation was that nothing was reported about the patient skin condition and general nutritional status, or about the complexity of the prior surgical procedure. In addition, the ALBC and PBL groups in three studies [12, 16, 26] contained larger proportions of patients with diabetes mellitus, a higher ASA rating, a slightly younger age, and diagnoses other than osteoarthritis. Many authors found that a greater comorbidity predicted a higher incidence of infection and perhaps ALBC would be beneficial in this high-risk group [1, 2, 13]. Moreover, a recent study stated that the use of ALBC in primary TKA might not be justified even in the group of patients considered as high risk [17].
Final limitation was that the sample size in two reports was larger than in the other studies [12, 16]. However, with or without these two studies, our overall pooled results revealed no significant decrease in the infection rate in ALBC-treated patients. In all studies there were no reports of toxicity or allergies attributed to the use of ALBCs. In addition, the bibliographic research was limited to published comparative studies (ALBC vs PBC) and did not take into account RCTs, RE studies and PRO trials in which there was no control group [5, 9, 18, 22, 27]. Thus, the conclusions of the present review are not absolute.
Although these limitations, the clinical relevance of this review was that use of ALBC did not significantly reduce the risk of infection in primary TKA. Moreover, concerns remain about the risks of hypersensitivity, or toxicity of antibiotics within cement, increased costs, impaired mechanical properties of the cement, and increased risk of selection of antibiotic-resistant organisms.
Conclusion
The findings of the present review did not reveal any statistically significant differences in terms of the rate of deep or superficial surgical site infections in patients receiving ALBC versus PBC. Although ALBC is worldwide frequently used, the periprosthetic knee infections continue to verify. However, the rigorous use of peri-operative prophylactic systemic antibiotics, efficient antiseptic procedures and improved surgical techniques remain the gold-standard in infection prevention in TKA surgery.
References
Bistolfi A, Massazza G, Verné E et al (2011) Antibiotic-loaded cement in orthopedic surgery: a review. ISRN Orthop 2011:290851
Bourne RB (2004) Prophylactic use of antibiotic bone cement: an emerging standard—in the affirmative. J Arthroplasty 19(4 Suppl 1):69–72
Buchholz HW, Engelbrecht H (1970) Depot effects of various antibiotics mixed with Palacos resins. Chirurg 41:511–515
Chiu FY, Chen CM, Lin CF et al (2002) Cefuroxime-impregnated cement in primary total knee arthroplasty: a prospective, randomized study of three hundred and forty knees. J Bone Joint Surg Am 84-A:759–962
Chiang CC, Chiu FY (2012) Cefuroxime-impregnated cement and systemic cefazolin for 1 week in primary total knee arthroplasty: an evaluation of 2700 knees. J Chin Med Assoc 75:167–170
Eveillard M, Mertl P, Tramier B et al (2003) Effectiveness of gentamicin-impregnated cement in the prevention of deep wound infection after primary total knee arthroplasty. Infect Control Hosp Epidemiol 24:778–780
Gandhi R, Razak F, Pathy R et al (2009) Antibiotic bone cement and the incidence of deep infection after total knee arthroplasty. J Arthroplasty 24:1015–1018
Gutowski CJ, Zmistowski BM, Clyde CT et al (2014) The economics of using prophylactic antibiotic-loaded bone cement in total knee replacement. Bone Joint J 96-B:65–69
Hansen EN, Adeli B, Kenyon R et al (2014) Routine use of antibiotic laden bone cement for primary total knee arthroplasty: impact on infecting microbial patterns and resistance profiles. J Arthroplasty 29:1123–1127
Hanssen AD (2004) Prophylactic use of antibiotic bone cement: an emerging standard – in opposition. J Arthroplasty 19(4 Suppl 1):73–77
Hanssen AD, Spangehl MJ (2004) Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacements. Clin Orthop Relat Res 427:79–85
Hinarejos P, Guirro P, Leal J et al (2013) The use of erythromycin and colistin-loaded cement in total knee arthroplasty does not reduce the incidence of infection: a prospective randomized study in 3000 knees. J Bone Joint Surg Am 95:769–774
Hinarejos P, Guirro P, Puig-Verdie L et al (2015) Use of antibiotic-loaded cement in total knee arthroplasty. Word J Orthop 6:877–885
Kurtz SM, Ong KL, Lau E et al (2010) Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 468:52–56
Malik MH, Chougle A, Pradhan N et al (2005) Primary total knee replacement: a comparison of a nationally agreed guide to best practice and current surgical technique as determined by the North West Regional Arthroplasty Register. Am R Coll Surg Engl 87:117–122
Namba RS, Chen Y, Paxton EW et al (2009) Outcome of routine use of antibiotic-loaded cement in primary total knee arthroplasty. J Arthroplasty 24(6 Suppl):44–47
Qadir R, Sidhu S, Ochsner JL et al (2014) Risk stratified usage of antibiotic-loaded bone cement for primary total knee arthroplasty: short term infection outcomes with a standardized cement protocol. J Arthroplasty 29:1622–1624
Randelli P, Evola R, Cabitza P et al (2009) L’uso profilattico del cemento antibiotato nei primi impianti protesici del ginocchio. GIOT 35:122–128
Randelli P, Evola FR, Cabitza P et al (2010) Prophylactic use of antibiotic-loaded bone cement in primary total knee replacement. Knee Surg Sports Traumatol Arthrosc 18:181–186
Robertsson O, Knutson K, Lewold S et al (2001) The Swedish Knee Arthroplasty Register 1975–1997: an update with special emphasis on 41,223 knees operated on in 1988–1997. Acta Orthop Scand 72:503–513
Shamseer L, Moher D, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 349:g7647
Srivastav AK, Nadkarni B, Srivastav S et al (2009) Prophylactic use of antibiotic-loaded bone cement in primary total knee arthroplasty: justified or not? Indian J Orthop 43:259–263
Tabulin J, D’Ollonne T, Cambas PM (2012) Antibiotic addition to cement—is it beneficial. Hip Int 22:9–12
Vasso M, Schiavone Panni A (2015) Low-grade periprosthetic knee infection: diagnosis and management. J Orthop Traumatol 16:1–7
Vasso M, Schiavone Panni A, De Martino I et al (2016) Prosthetic knee infection by resistant bacteria: the worst-case scenario. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-016-4010-8
Wang H, Qiu G, Lin J et al (2014) Antibiotic bone cement cannot reduce deep infection after primary total knee arthroplastly. Orthopaedics 38:e462–e466
Wu CT (2016) Surgical site infection after total knee arthroplasty: risk factors in patient with timely administration of systemic prophylactic antibiotics. J Arthroplasty. doi:10.1016/j.arth.2016.01.017
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authours declares that they have no conflict of interest.
Funding
No funding.
Ethical approval
All procedures performed in studies involving human partecipants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinski declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study format consent is not required.
Rights and permissions
About this article
Cite this article
Schiavone Panni, A., Corona, K., Giulianelli, M. et al. Antibiotic-loaded bone cement reduces risk of infections in primary total knee arthroplasty? A systematic review. Knee Surg Sports Traumatol Arthrosc 24, 3168–3174 (2016). https://doi.org/10.1007/s00167-016-4301-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-016-4301-0