Abstract
Purpose
Although obesity has historically been described as a contraindication to UKA, improved outcomes with modern UKA implant designs have challenged this perception. The purpose of this study was to assess the influence of obesity on the outcomes of UKA with a robotic-assisted system at a minimum follow-up of 24 months with the hypothesis that obesity has no effect on robotic-assisted UKA outcomes.
Methods
There were 746 medial robotic-assisted UKAs (672 patients) with a mean age of 64 years (SD 11) and a mean follow-up time of 34.6 months (SD 7.8). Mean overall body mass index (BMI) was 32.1 kg/m2 (SD 6.5), and patients were stratified into seven weight categories according to the World Health Organization classification.
Results
Patient BMI did not influence the rate of revision surgery to TKA (5.8 %) or conversion from InLay to OnLay design (1.7 %, n.s.). Mean postoperative Oxford knee score was 37 (SD 11) without correlation with BMI (n.s.). The type of prosthesis (InLay/OnLay) regardless of BMI had no influence on revision rate (n.s.). BMI did not influence 90-day readmissions (4.4 %, n.s.), but showed significant correlation with higher opioid medication requirements and a higher number of physical therapy session needed to reach discharge goals (p = 0.031).
Conclusion
These findings suggest that BMI does not influence clinical outcomes and readmission rates of robotic-assisted UKA at mid-term. The classic contraindication of BMI >30 kg/m2 may not be justified with the use of modern UKA designs or techniques.
Level of evidence
IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unicompartmental knee arthroplasty (UKA) has gained renewed interest in the past decade as an alternative to total knee arthroplasty (TKA) for selected patients with degenerative joint disease limited to the lateral or medial compartment of the knee [4, 33, 36]. The advantages of UKA over TKA are reduced blood loss, less perioperative morbidity, faster recovery and rehabilitation, as well as increased postoperative range of motion compared to total knee arthroplasty [6, 10, 17, 26, 34, 42]. Historically, obesity was thought to result in suboptimal clinical outcomes and increased revision rates following conventional UKA using manual instrumentation [5, 6, 24]. More recent studies have continued to support obesity as a contraindication to UKA. In a consecutive patient series, Bonutti et al. [9] found lower implant survival rates in patients with BMI >35 at a minimum 2-year follow-up. Kandil et al. [27] reviewed a large national database including 1823 obese and 1019 morbidly obese patients and found increased medical complications and early revision rates compared to non-obese patients. However, the concurrent literature in recent years has challenged the indications for UKA to include patients with a higher BMI [11, 12, 39]. At an average 12-year follow-up, Cavaignac et al. [11] found no difference in UKA survival rates between patients with BMIs over or under 32. Murray et al. [39] assessed outcomes in 2438 mobile-bearing UKAs stratified by patient BMI and found no association between failure rate and BMI at mean 5-year follow-up.
The impact of obesity on the outcomes of robotic-assisted UKA has not been assessed. Robotic-assisted systems are available for UKA and have been shown to improve component positioning compared to conventional manual UKA procedures [15, 17, 31, 35, 43, 48]. UKA is technically challenging, and precise component placement is vital for implant survival [3, 17, 32, 36, 37, 47, 55], where over- or under-correction by as little as 2° may lead to improper component placement, possibly increasing polyethylene wear of the tibial component, leading to progression of osteoarthritic changes in other compartments of the knee, or causing anterior knee pain [3, 16, 17, 25, 34, 37, 41, 42, 46]. In cadaveric and clinical studies, robotic-assisted UKA was found to have less variability in implant position compared to manual component insertion by the same surgeon [14, 35]. In addition, robotic-assisted UKA provides an objective method to perform accurate soft tissue balancing during the procedure [45]. The improved component positioning accuracy afforded by robotic systems may be particularly advantageous in obese patients with increased stresses at the bone–implant interface [20].
The purpose of this study was to assess the influence of obesity on the outcomes of UKA with a robotic-assisted system at a single institution. We hypothesized that increased body mass index (BMI) does not influence the outcomes of robotic-assisted UKA and that outcomes are comparable to conventional UKA recorded in various national joint registries.
Materials and methods
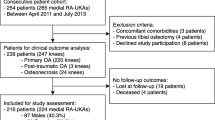
The joint registry at a single university medical centre was analysed for patients who underwent robotic-assisted UKA (MAKO Surgical Corp., Ft Lauderdale, FL) between 2008 and 2012 by four surgeons. Current author indication included patients generally older than 40 years of age, medium to high preoperative activity levels, no anterior cruciate ligament instability or pivot shift, less than 15 degrees of varus deformity and less than 15 degrees of flexion contracture, and no weight restrictions. The implants consisted of cemented, fixed-bearing femoral and tibial components with InLay or OnLay based on surgeon preference. Patients who received a medial compartment UKA and had a minimum of 24 months of follow-up were stratified according to BMI. There were seven weight categories based on a clinical modification of the World Health Organization classification system [56].
Patient demographics including age, Charlson comorbidity index, American Society of Anesthesiologists physical status classification system (ASA), length of hospitalization and Oxford knee score at final follow-up were retrieved from patients’ medical records. All patient medical records were assessed for length of surgery (incision to closure time), 90-day mortality and readmissions, postoperative complications, and revision surgery. Patients’ opioid pain medication requirements for the first 3 days after surgery and the number of physical therapy sessions required to reach discharge goals were recorded for each patient. The amount of oral and intravenous opioid pain medication was converted to morphine equivalent doses (MED) based on available conversion tables [13, 44]. Patients were followed in clinic at 2 months, 6 months and 1 year after surgery and then annually. Patients who were not seen in clinic for more than 6 months (27 %) from the time of this investigation were contacted by phone to acquire Oxford knee scores and inquire about possible revision surgery. Institutional Review Board approval from Wake Forest Baptist Health was obtained prior to the beginning of this study (IRB 2873).
Statistical analysis
Data are reported as the mean ± standard deviation (SD) for continuous variables and as percentages for categorical variables. Chi-square analysis was used to assess the influence of BMI on postoperative complications, revision surgeries and 90-day readmissions. Correlation analysis was used to analyse the influence of BMI on length of surgery and hospitalization. Statistical analysis was performed with alpha 0.05 (Prism 6, GraphPad, LaJolla, CA, USA).
Results
A total of 1032 medial robotic-assisted UKAs were performed in the study period. A total of 746 UKAs (672 patients) had >24 months of follow-up and were included in this study (Table 1). There were 595 InLay prosthesis and 151 OnLay designs. There was no correlation between length of surgery and BMI (n.s.) or length of hospitalization and BMI (n.s.). There was a significant correlation between increasing BMI and higher ASA score (p < 0.001), but there was no correlation between Charlson comorbidity index and BMI (p = 0.096; Table 2).
Opioid medication requirement was highest following surgery including postoperative day 1 (28.3 ± 25.0 MED, n = 743) and significantly decreased (p < 0.05) on postoperative day 2 (20.5 ± 22.1 MED, n = 472). Opioid requirements were similar on postoperative day 3 (16.1 ± 19.2 MED, n = 118). There was a significant correlation between increasing BMI and higher opioid medication requirements following surgery including on postoperative day 1 (p = 0.002), on postoperative day 2 (p = 0.007) and on postoperative day 3 (p = 0.007). Patients required a mean of 2.1 ± 1.3 physical therapy sessions to reach discharge goals which correlated with increasing BMI (p = 0.031). The mean Oxford knee score at final follow-up was 37 ± 11 (range 2–48) and did not correlate with patient’s BMI (p = 0.387).
Patient’s BMI did not influence the rate of revision surgery to TKA or conversion from InLay to OnLay design (n.s.) (Table 3). There were 43 revisions to total knee arthroplasty (5.8 %) in the entire group (Table 4). Of 595 InLay prostheses, 11 were converted from an InLay to an OnLay design (1.8 %; Table 4); two patients subsequently underwent revision to TKA. The type of prosthesis (InLay/OnLay) regardless of patient BMI had no influence on revision rate to TKA (n.s.) or all revision surgery (n.s.). Two patients received a robotic-assisted patellofemoral arthroplasty during revision from InLay to OnLay design. One patient with an InLay prosthesis (0.1 %) and another patient with an OnLay prosthesis (0.1 %) underwent open arthrotomy for removal of cement and osteophytes causing mechanical symptoms and pain.
There were four infections (0.5 %) in this study in patients with BMIs ranging from 23.6 to 29.8. Two patients with an OnLay prosthesis underwent open irrigation and debridement with polyethylene liner exchange which resolved the infection. One patient with an InLay prosthesis underwent open irrigation and debridement and received intravenous antibiotics which resolved the infection. Another patient with an InLay prosthesis underwent arthroscopic irrigation and debridement and subsequently required revision to TKA.
A total of 37 patients (5.0 %) in this study underwent arthroscopy following robotic-assisted UKA: 36 for pain and mechanical symptoms (97 %) and one for infection (3 %). BMI was not associated with the need for arthroscopy (n.s.); however, all arthroscopies were in patients who received an InLay prosthesis (p < 0.001). Two patients subsequently required conversion from an InLay to OnLay design, and two patients required revision to TKA.
The femoral component design was changed by the manufacturer during the course of this investigation. Of all 43 revisions to TKA, 21 patients (49 %) had received a femoral component with one peg and 22 patients (51 %) had received a newer component with two pegs.
There were a total of 34 readmissions (4.4 %) that occured within 90 days after surgery without correlation with BMI (n.s.) (Table 5).
Discussion
The most important finding of the current study was that BMI had no influence on the short-term to mid-term clinical outcomes and readmission rates of patients who underwent medial robotic-assisted UKA. This study is the first to assess the effects of BMI on outcomes after robotic-assisted UKA, and these findings concur with previous studies assessing the influence of BMI on conventional UKA, which found no association between BMI and failure rate [39, 50]. The revision rate of the current study is similar to the reported revision rates of national registries [2, 40]. Therefore, the classic contraindication of a BMI >30 kg/m2 may not be justified with the use of robotic-assisted UKA designs. Furthermore, BMI had no effect on length of hospital stay, length of surgery or 90-day readmission rate. However, increased BMI was associated with increased narcotic pain medication requirements and utilization of physical therapy resources prior to discharge.
The outcomes of UKA with early component designs were shown to be influenced by patient BMI, and as a result, a weight restriction of 82 kg was recommended by Kozinn and Scott [29] to avoid early prosthesis failure. Deshmukh and Scott [11, 21] increased this weight restriction to 90 kg. In an analysis of 73 medial UKAs with a fixed-bearing design in 61 patients with a minimum follow-up of 2 years, BMI of >32 was identified as a causative factor for early failure [5]. Bonutti et al. [9] compared fixed-bearing UKAs in 34 patients (40 UKAs) with a BMI of ≥35 kg/m2 with 33 patients (40 UKAs) who had a BMI of <35 kg/m2. Obese patients had a 12.5 % higher failure rate after a minimum follow-up of 24 months compared with non-obese patients [9]. Failures resulted from progression of painful arthritis, tibial component loosening and intractable pain which the authors attributed to improper component alignment [9]. The most common reason for revision to TKA in the current study was intractable pain (46 %) and tibial component loosening (12 %). However, there was no association between BMI and reason for failure in the current study (p < 0.05).
The current literature is equivocal for outcomes following fixed- and mobile-bearing UKA designs. Fixed-bearing designs appear to have improved long-term survivorship with more consistent results and are technically less challenging to perform [8]. However, newer mobile-bearing designs, while more challenging to perform, may deliver promising results in patients with higher BMI’s secondary to the prosthesis maintaining more native joint mechanics that preserve adequate contact between UKA components throughout range of motion to reduce pressure and bone-prosthesis stress [18, 23, 30, 53]. In an analysis of 2438 UKAs with a mobile-bearing prosthesis at a mean follow-up of 5 years, BMI did not influence the revision rate [39]. The authors concluded that BMI as a surgical limitation should be abandoned [39]. In a study of 100 UKAs in 82 patients stratified according to BMI and followed over a period of 20 years, obese patients had a decreased revision rate [52]. Emerson et al. [22] observed a decreased revision rate in mobile-bearing designs in patients with increased BMI and concluded that tibial component failure may be reduced in mobile-bearing UKAs. The findings of the current study with a fixed tibial component suggest that patient BMI does not influence outcomes; however, long-term follow-up with robotic-assisted fixed-bearing designs will be needed.
The current literature is ambivalent about which tibial component design is most efficacious in obese patients. The tibial resection is more extensive for an OnLay design, while the all-polyethylene InLay design preserves tibial bone. However, the advantage of decreased bone resection with the InLay design might be negated by increased interface stresses between the polyethylene insert and tibial bone surface, which may be increased in obese patients based on the axial force across the interface [49, 54]. Previous studies revealed improved mechanical alignment with an OnLay design which could prolong implant survival [19, 51]. In a study of 1746 UKAs, OnLay components were found to have a decreased revision rate at a median of 3.6 years [7]. However, Murray et al. [38] compared 207 InLay to 202 OnLay UKAs and found similar revision rates. Aleto et al. [1] retrospectively analysed failed UKAs and reported premature failure in all-polyethylene tibial implants. While various reports state that the difference between the failure rates of OnLay and InLay appeared to be significant even after BMI was adjusted, some studies suggest that there is no difference. In the current study, the type of prosthesis, regardless of patient BMI, had no statistically significant influence on revision rate (p = 0.069). However, a trend can be noted towards InLay prosthesis having a higher number of revision surgeries including revision to TKA and conversion of an InLay to OnLay design. In addition, there were a significantly higher number of patients with an InLay component that required arthroscopy following UKA for pain and mechanical symptoms.
The overall revision rate of UKA to TKA in the current study is comparable (p = 0.785) to reported revision rates of conventional UKA in national registries at 3-year follow-up (4.7 % to approx. 6.5 %, Table 6). However, specific designs are typically not reported in these registries limiting their application to this comparison. Future studies will need to assess the effect of precise component positioning and ligament balancing using a robotic-assisted system on the long-term outcomes UKA in patients with elevated BMI.
This study has various limitations that need to be considered when interpreting the findings. The majority of UKAs in this study were performed by one surgeon (60 %) and were mainly InLay design UKAs (96 %), while the OnLay design UKAs were performed by three other surgeons (70; 23; 7 %). Surgeon experience was found to be a key factor for UKA success. In a study comparing 72 InLay with 75 OnLay UKAs, surgeon experience (more than 50 UKA/year vs. <10 UKA/year) and correct component positioning were determining factors for implant survival, not the implant design [57]. Robotic-assisted surgery decreases the learning curve for surgeons, which may reduce the effect of surgeon experience on implant survivorship [28]. In combination with improved component positioning, surgeons with limited experience performing UKA may benefit from robotic-assisted surgery. Furthermore, the majority of UKAs in this study were InLays (80 %), while the most commonly used UKA design is OnLay, limiting the generalizability of the results of this study. The femoral component design was changed by the manufacturer during the course of this investigation. However, there was no difference in the number of revision surgeries to TKA between the two designs. This study does not include a radiographic analysis of the failure mechanism of robotic-assisted medial UKA, which will be addressed in future studies. The mean short-term follow-up time of this study was 36 months. Long-term studies will be needed to evaluate the success of robotic-assisted fixed-bearing UKAs compared to conventional mobile-bearing UKAs.
Conclusion
Based on the findings of the current study, BMI had no influence on the clinical outcomes, length of hospital stay or readmission rates of patients who underwent medial robotic-assisted UKA with short-term follow-up. However, increasing BMI was associated with higher hospital resource utilization and narcotic pain medication requirements. Obesity may not be justified as a contraindication to UKA.
References
Aleto TJ, Berend ME, Ritter MA, Faris PM, Meneghini RM (2008) Early failure of unicompartmental knee arthroplasty leading to revision. J Arthroplasty 23(2):159–163
Australian Orthopaedic Association National Joint Replacement Registry (2014). Annual Report. https://aoanjrr.dmac.adelaide.edu.au/en/annual-reports-2014
Banks SA, Harman MK, Hodge WA (2002) Mechanism of anterior impingement damage in total knee arthroplasty. J Bone Joint Surg Am 84-A(Suppl 2):37–42
Beard DJ, Pandit H, Gill HS, Hollinghurst D, Dodd CA, Murray DW (2007) The influence of the presence and severity of pre-existing patellofemoral degenerative changes on the outcome of the Oxford medial unicompartmental knee replacement. J Bone Joint Surg Br 89(12):1597–1601
Berend KR, Lombardi AV Jr, Mallory TH, Adams JB, Groseth KL (2005) Early failure of minimally invasive unicompartmental knee arthroplasty is associated with obesity. Clin Orthop Relat Res 440:60–66
Bert JM (2005) Unicompartmental knee replacement. Orthop Clin North Am 36(4):513–522
Bini S, Khatod M, Cafri G, Chen Y, Paxton EW (2013) Surgeon, implant, and patient variables may explain variability in early revision rates reported for unicompartmental arthroplasty. J Bone Joint Surg Am 95(24):2195–2202
Bonutti PM, Dethmers DA (2008) Contemporary unicompartmental knee arthroplasty: fixed vs mobile bearing. J Arthroplasty 23(7 Suppl):24–27
Bonutti PM, Goddard MS, Zywiel MG, Khanuja HS, Johnson AJ, Mont MA (2011) Outcomes of unicompartmental knee arthroplasty stratified by body mass index. J Arthroplasty 26(8):1149–1153
Borus T, Thornhill T (2008) Unicompartmental knee arthroplasty. J Am Acad Orthop Surg 16(1):9–18
Cavaignac E, Lafontan V, Reina N, Pailhe R, Wargny M, Laffosse JM, Chiron P (2013) Obesity has no adverse effect on the outcome of unicompartmental knee replacement at a minimum follow-up of seven years. Bone Joint J 95-B(8):1064–1068
Cepni SK, Arslan A, Polat H, Yalcin A, Parmaksizoglu AS (2014) Mid-term results of Oxford Phase 3 unicompartmental knee arthroplasty in obese patients. Acta Orthop Traumatol Turc 48(2):122–126
Cherny NI (1996) Opioid analgesics: comparative features and prescribing guidelines. Drugs 51(5):713–737
Citak M, Suero EM, Dunbar NJ, Branch SH, Conditt MA, Banks SA, Pearle AD (2013) Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee 20(4):268–271
Cobb J, Henckel J, Gomes P, Harris S, Jakopec M, Rodriguez F, Barrett A, Davies B (2006) Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br 88(2):188–197
Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA (2006) Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty 21(6 Suppl 2):108–115
Conditt MA, Roche MW (2009) Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am 91(Suppl 1):63–68
Confalonieri N, Manzotti A, Pullen C (2004) Comparison of a mobile with a fixed tibial bearing unicompartmental knee prosthesis: a prospective randomized trial using a dedicated outcome score. Knee 11(5):357–362
Cossey AJ, Spriggins AJ (2005) The use of computer-assisted surgical navigation to prevent malalignment in unicompartmental knee arthroplasty. J Arthroplasty 20(1):29–34
Dalury DF, Tucker KK, Kelley TC (2012) All-polyethylene tibial components in obese patients are associated with low failure at midterm followup. Clin Orthop Relat Res 470(1):117–124
Deshmukh RV, Scott RD (2001) Unicompartmental knee arthroplasty: long-term results. Clin Orthop Relat Res 392:272–278
Emerson RH Jr, Hansborough T, Reitman RD, Rosenfeldt W, Higgins LL (2002) Comparison of a mobile with a fixed-bearing unicompartmental knee implant. Clin Orthop Relat Res 404:62–70
Goodfellow JW, O’Connor J (1986) Clinical results of the Oxford knee. Surface arthroplasty of the tibiofemoral joint with a meniscal bearing prosthesis. Clin Orthop Relat Res 205:21–42
Heck DA, Marmor L, Gibson A, Rougraff BT (1993) Unicompartmental knee arthroplasty. A multicenter investigation with long-term follow-up evaluation. Clin Orthop Relat Res 286:154–159
Hernigou P, Deschamps G (2004) Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res 423:161–165
Jenny JY, Ciobanu E, Boeri C (2007) The rationale for navigated minimally invasive unicompartmental knee replacement. Clin Orthop Relat Res 463:58–62
Kandil A, Werner BC, Gwathmey WF, Browne JA (2015) Obesity, morbid obesity and their related medical comorbidities are associated with increased complications and revision rates after unicompartmental knee arthroplasty. J Arthroplasty 30(3):456–460
Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J (2013) Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop 2013:481039
Kozinn SC, Scott R (1989) Unicondylar knee arthroplasty. J Bone Joint Surg Am 71(1):145–150
Kwon OR, Kang KT, Son J, Kwon SK, Jo SB, Suh DS, Choi YJ, Kim HJ, Koh YG (2014) Biomechanical comparison of fixed- and mobile-bearing for unicompartmental knee arthroplasty using finite element analysis. J Orthop Res 32(2):338–345
Lang JE, Mannava S, Floyd AJ, Goddard MS, Smith BP, Mofidi A, Seyler TM, Jinnah RH (2011) Robotic systems in orthopaedic surgery. J Bone Joint Surg Br 93(10):1296–1299
Li G, Papannagari R, Most E, Park SE, Johnson T, Tanamal L, Rubash HE (2005) Anterior tibial post impingement in a posterior stabilized total knee arthroplasty. J Orthop Res 23(3):536–541
Lombardi AV, Berend KR, Berend ME, Della Valle CJ, Engh GA, Fitz W, Hurst JM, Jinnah RH, Lonner JH, Macaulay WB, Repicci JA, Scuderi GR (2012) Current controversies in partial knee arthroplasty. Instr Course Lect 61:347–381
Lonner JH (2009) Indications for unicompartmental knee arthroplasty and rationale for robotic arm-assisted technology. Am J Orthop (Belle Mead NJ) 38(2 Suppl):3–6
Lonner JH, John TK, Conditt MA (2010) Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res 468(1):141–146
Maduekwe UI, Zywiel MG, Bonutti PM, Johnson AJ, Delanois RE, Mont MA (2010) Scientific evidence for the use of modern unicompartmental knee arthroplasty. Expert Rev Med Devices 7(2):219–239
Mariani EM, Bourne MH, Jackson RT, Jackson ST, Jones P (2007) Early failure of unicompartmental knee arthroplasty. J Arthroplasty 22(6 Suppl 2):81–84
Murray DW, MacLennan GS, Breeman S, Dakin HA, Johnston L, Campbell MK, Gray AM, Fiddian N, Fitzpatrick R, Morris RW, Grant AM, Group KAT (2014) A randomised controlled trial of the clinical effectiveness and cost-effectiveness of different knee prostheses: the knee arthroplasty trial (KAT). Health Technol Assess 18(19):1–235
Murray DW, Pandit H, Weston-Simons JS, Jenkins C, Gill HS, Lombardi AV, Dodd CA, Berend KR (2013) Does body mass index affect the outcome of unicompartmental knee replacement? Knee 20(6):461–465
The Norwegian Arthroplasty Register, Helse-Bergen HF (2010) Department of Orthopaedic Surgery, Haukeland University Hospital. http://nrlweb.ihelse.net/eng/Report_2010.pdf
Park SE, Lee CT (2007) Comparison of robotic-assisted and conventional manual implantation of a primary total knee arthroplasty. J Arthroplasty 22(7):1054–1059
Pearle AD, Kendoff D, Musahl V (2009) Perspectives on computer-assisted orthopaedic surgery: movement toward quantitative orthopaedic surgery. J Bone Joint Surg Am 91(Suppl 1):7–12
Pearle AD, O’Loughlin PF, Kendoff DO (2010) Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty 25(2):230–237
Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E (2001) Equianalgesic dose ratios for opioids: a critical review and proposals for long-term dosing. J Pain Symptom Manag 22(2):672–687
Plate JF, Mofidi A, Mannava S, Smith BP, Lang JE, Poehling GG, Conditt MA, Jinnah RH (2013) Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop 2013:837167
Ridgeway SR, McAuley JP, Ammeen DJ, Engh GA (2002) The effect of alignment of the knee on the outcome of unicompartmental knee replacement. J Bone Joint Surg Br 84(3):351–355
Roche M, O’Loughlin PF, Kendoff D, Musahl V, Pearle AD (2009) Robotic arm-assisted unicompartmental knee arthroplasty: preoperative planning and surgical technique. Am J Orthop (Belle Mead NJ) 38(2 Suppl):10–15
Roche MW, Augustin D, Conditt MA (2010) One year outcomes of robotically guided UKA. J Bone Joint Surg Br 92-B(Supp 1):156–157
Scott CE, Eaton MJ, Nutton RW, Wade FA, Pankaj P, Evans SL (2013) Proximal tibial strain in medial unicompartmental knee replacements: A biomechanical study of implant design. Bone Joint J 95-B(10):1339–1347
Sebilo A, Casin C, Lebel B, Rouvillain JL, Chapuis S, Bonnevialle P, members of the Societe d’Orthopedie et de Traumatologie de lO (2013) Clinical and technical factors influencing outcomes of unicompartmental knee arthroplasty: retrospective multicentre study of 944 knees. Orthop Traumatol Surg Res 99(4 Suppl):S227–S234
Suero EM, Citak M, Njoku IU, Pearle AD (2013) Does the type of tibial component affect mechanical alignment in unicompartmental knee replacement? Technol Health Care 21(1):81–85
Tabor OB Jr, Tabor OB, Bernard M, Wan JY (2005) Unicompartmental knee arthroplasty: long-term success in middle-age and obese patients. J Surg Orthop Adv 14(2):59–63
Vorlat P, Putzeys G, Cottenie D, Van Isacker T, Pouliart N, Handelberg F, Casteleyn PP, Gheysen F, Verdonk R (2006) The oxford unicompartmental knee prosthesis: an independent 10-year survival analysis. Knee Surg Sports Traumatol Arthrosc 14(1):40–45
Walker PS, Parakh DS, Chaudhary ME, Wei CS (2011) Comparison of interface stresses and strains for onlay and inlay unicompartmental tibial components. J Knee Surg 24(2):109–115
Whiteside LA (2005) Making your next unicompartmental knee arthroplasty last: three keys to success. J Arthroplasty 20(4 Suppl 2):2–3
World Health Organization. Obesity: preventing and managing the global epedemic. Report of a WHO Consultation (2000). WHO technical report series 894
Zambianchi F, Digennaro V, Giorgini A, Grandi G, Fiacchi F, Mugnai R, Catani F (2014) Surgeon’s experience influences UKA survivorship: a comparative study between all-poly and metal back designs. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-014-2958-9
Conflict of interest
The authors JFP, MAA, TMS, DNB, AH and MA report no conflict of interest. The authors RHJ and GGP have received financial support from MAKO Surgical Corp., Ft Lauderdale, FL. RHJ and GGP have received payment as consultants. All authors certify that this investigation was performed in conformity with ethical principles of research. Institutional review board approval was obtained prior to the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Johannes F. Plate and Marco A. Augart have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Plate, J.F., Augart, M.A., Seyler, T.M. et al. Obesity has no effect on outcomes following unicompartmental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 25, 645–651 (2017). https://doi.org/10.1007/s00167-015-3597-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-015-3597-5